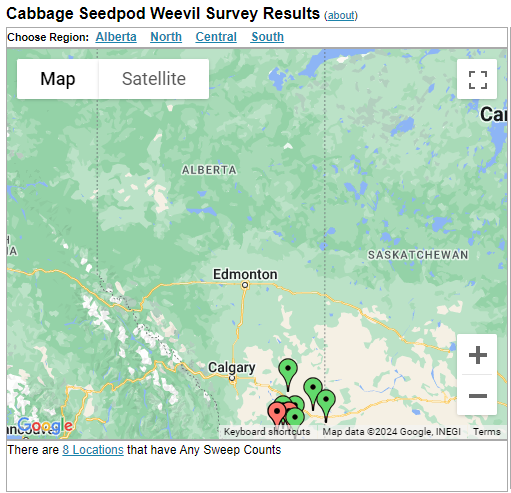
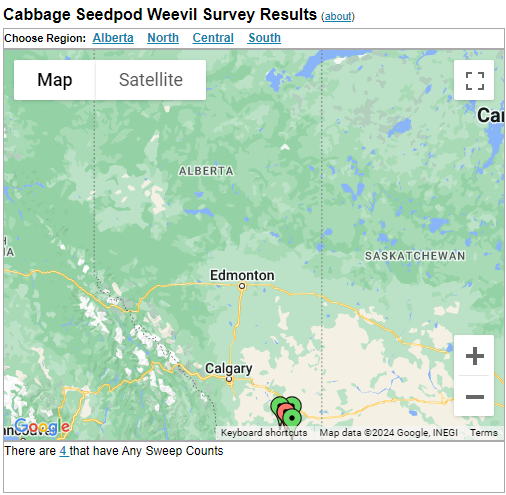
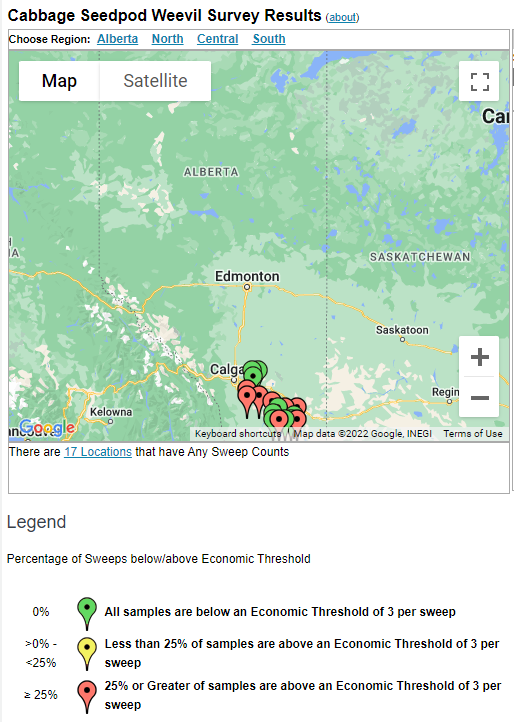
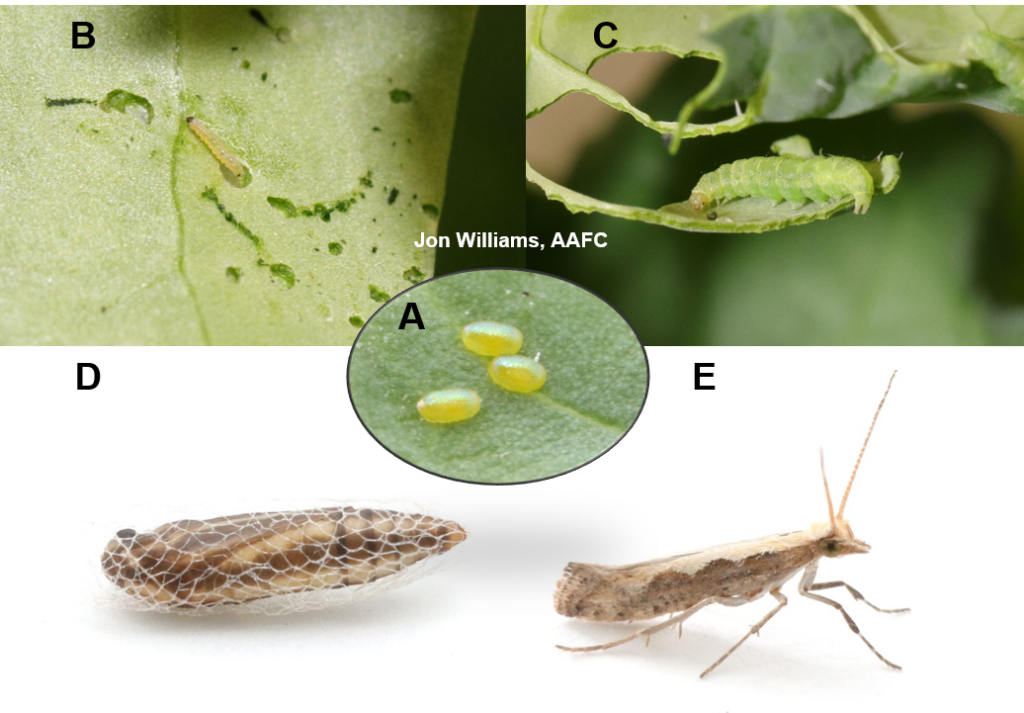
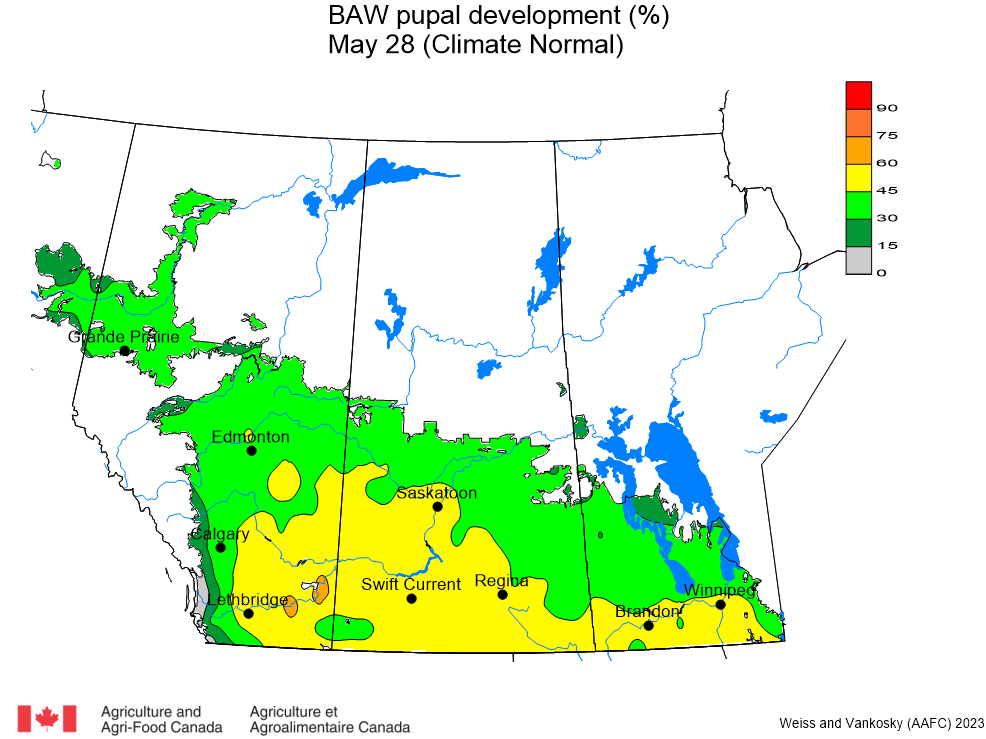
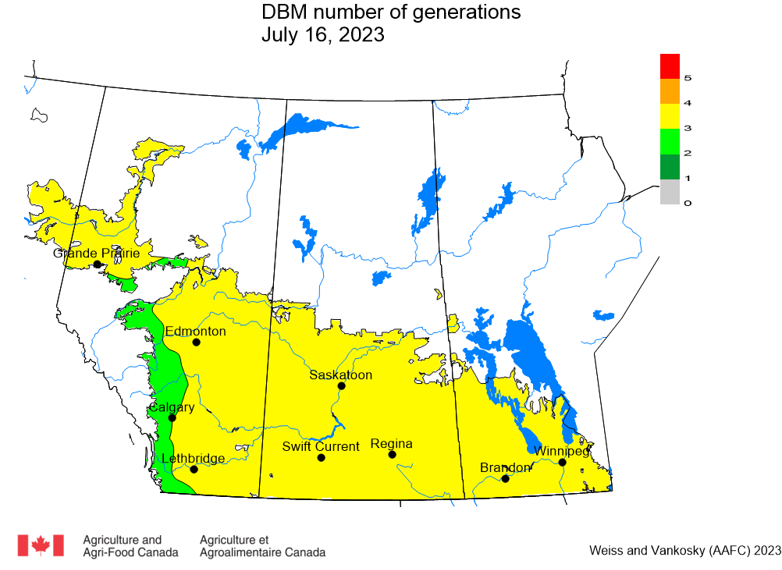
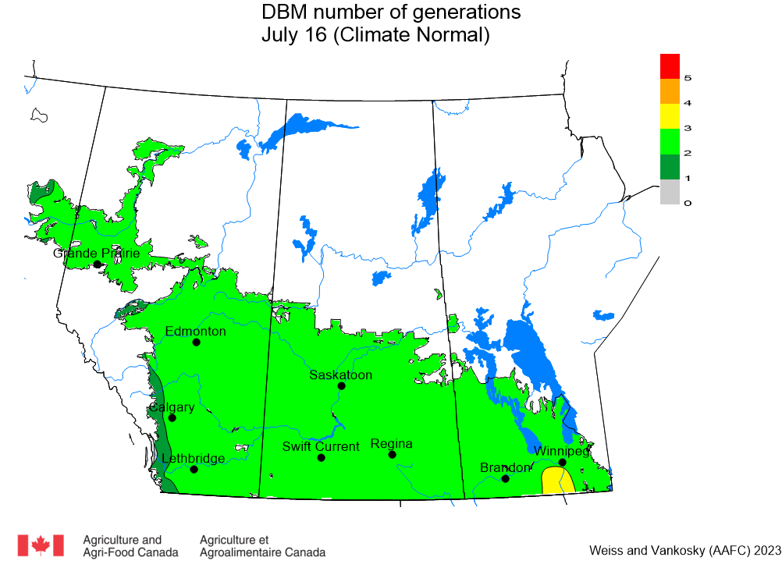
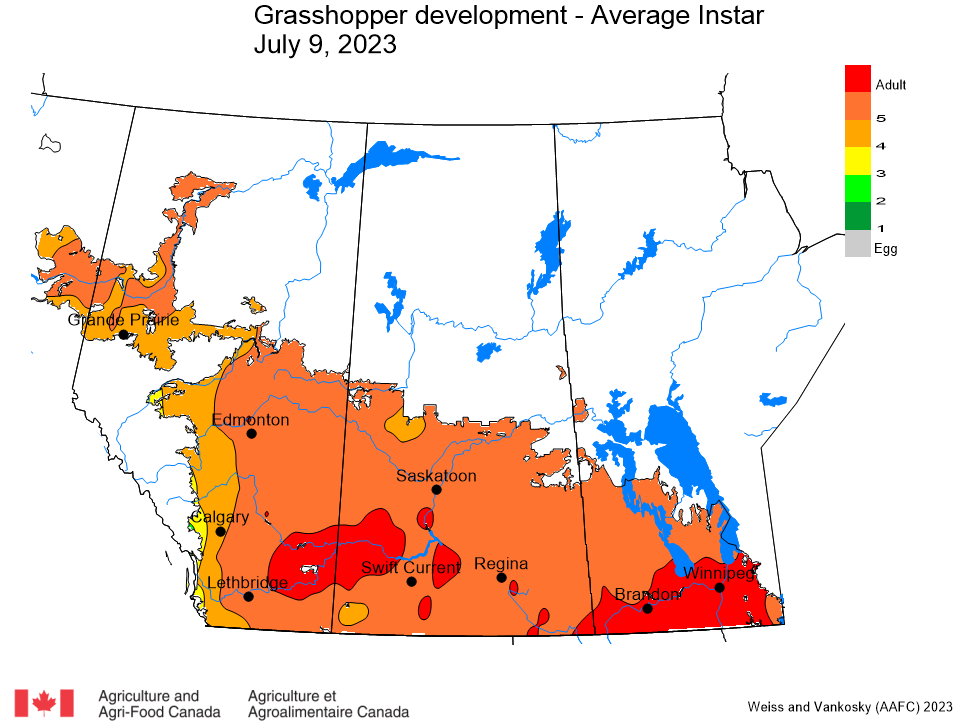
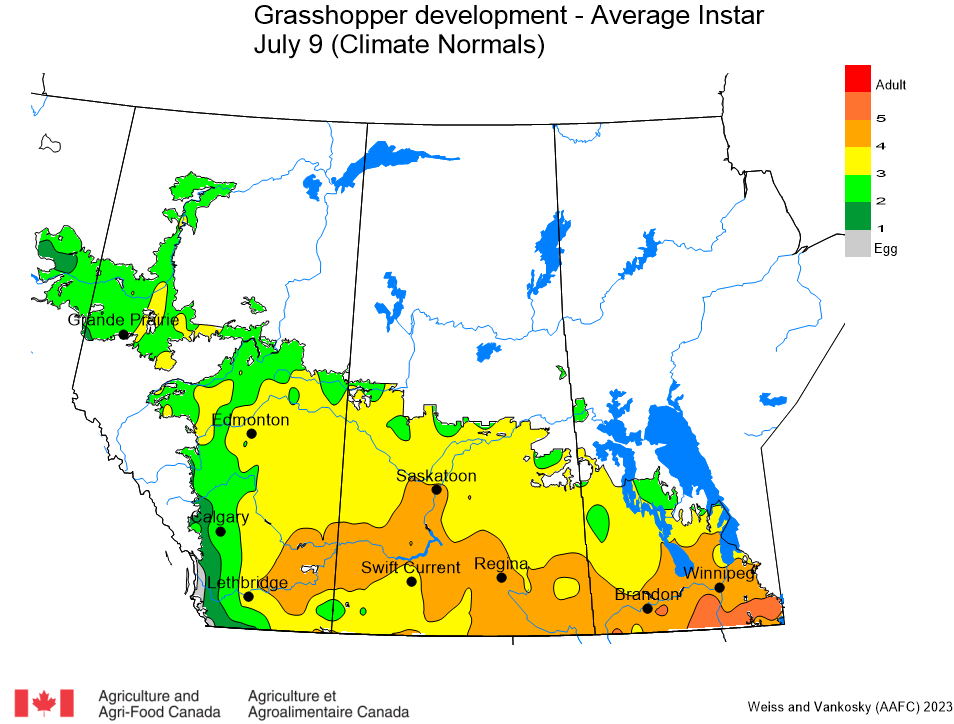
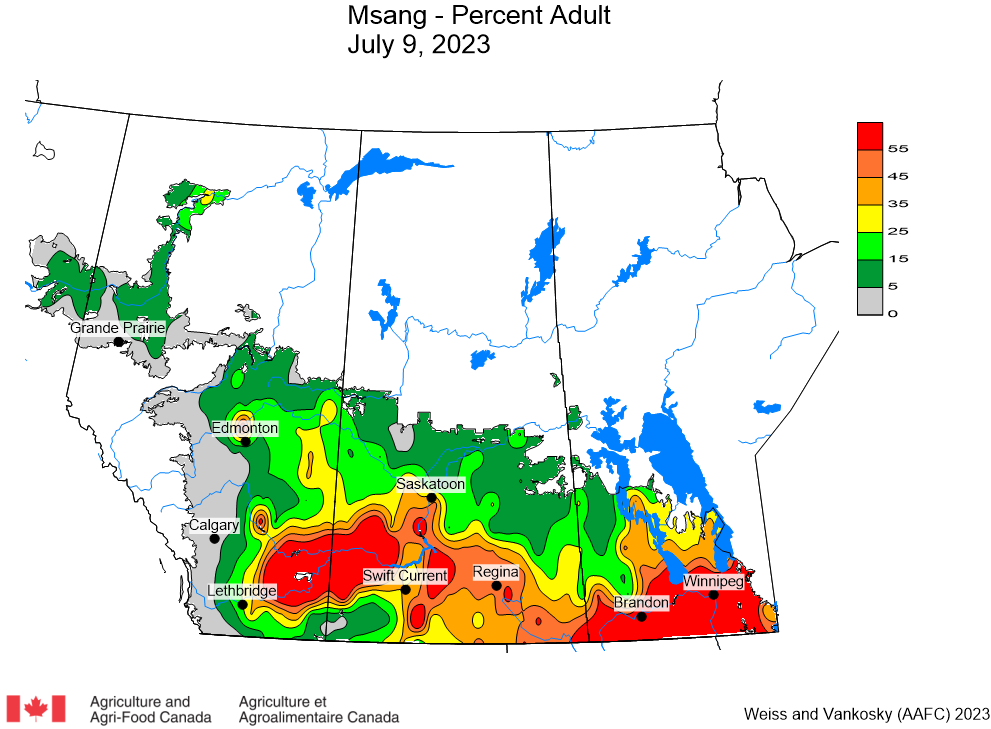
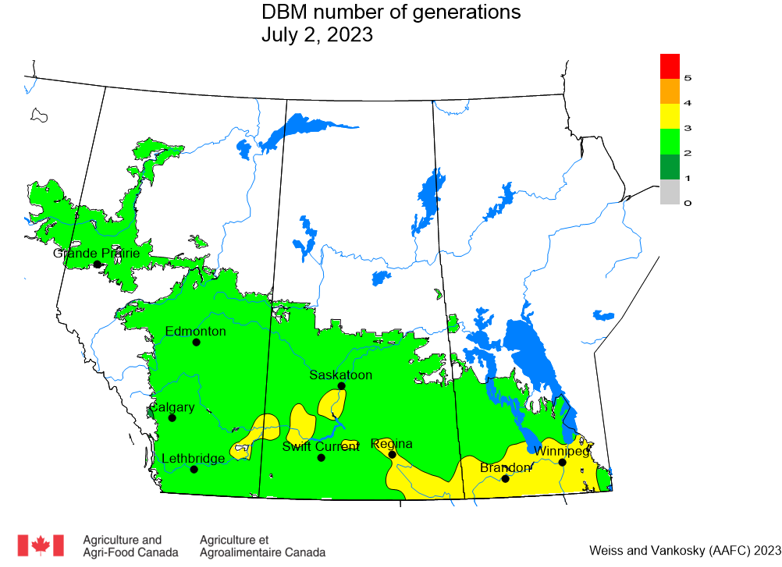
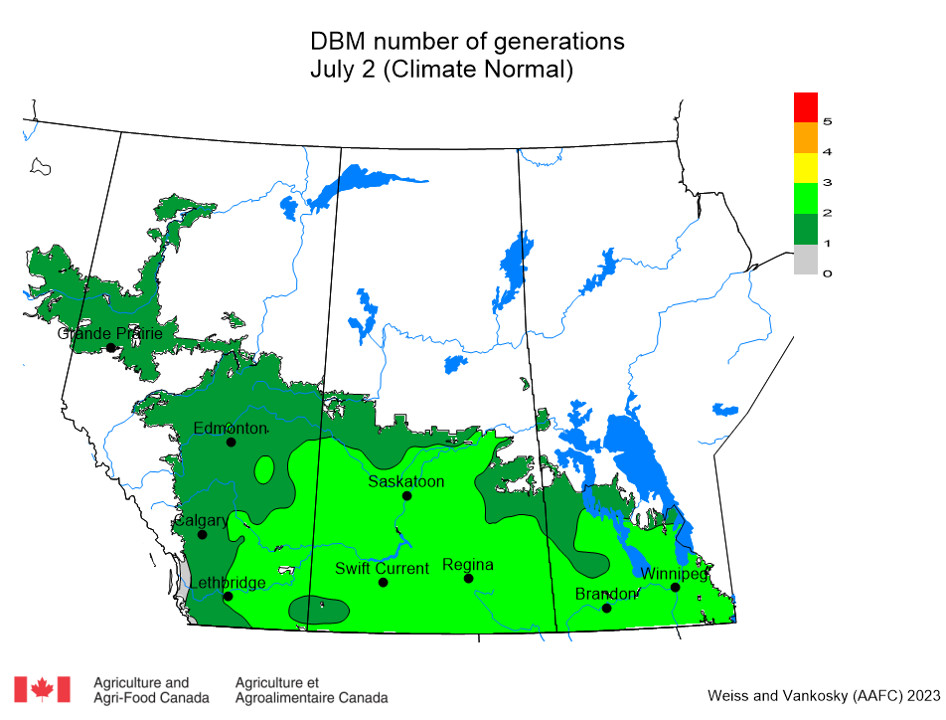
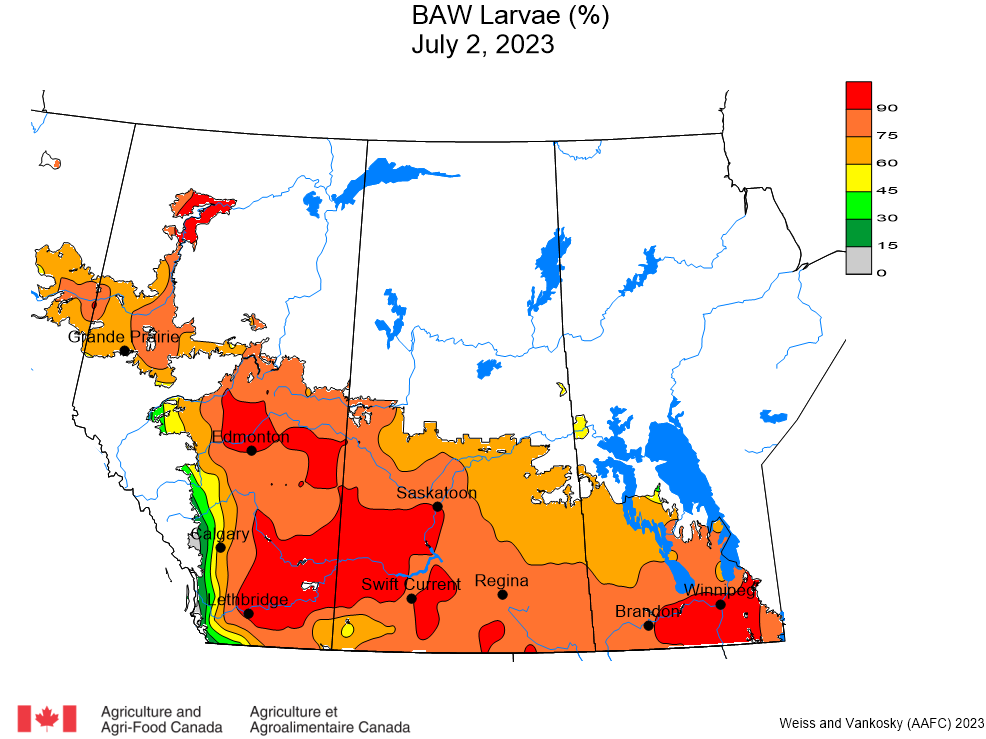
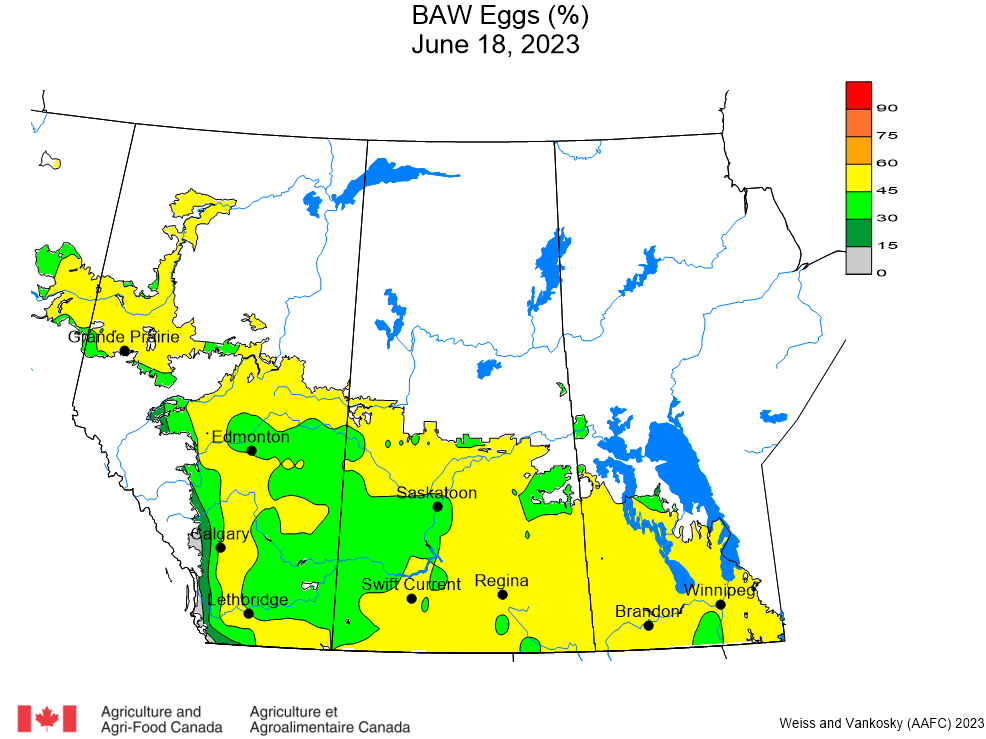
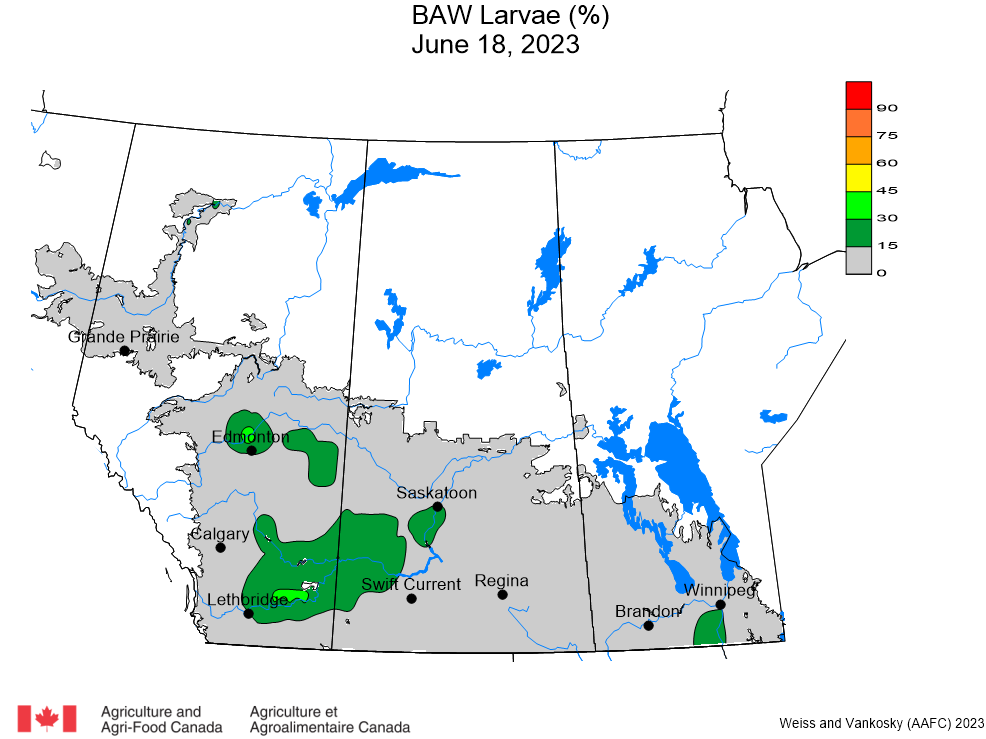
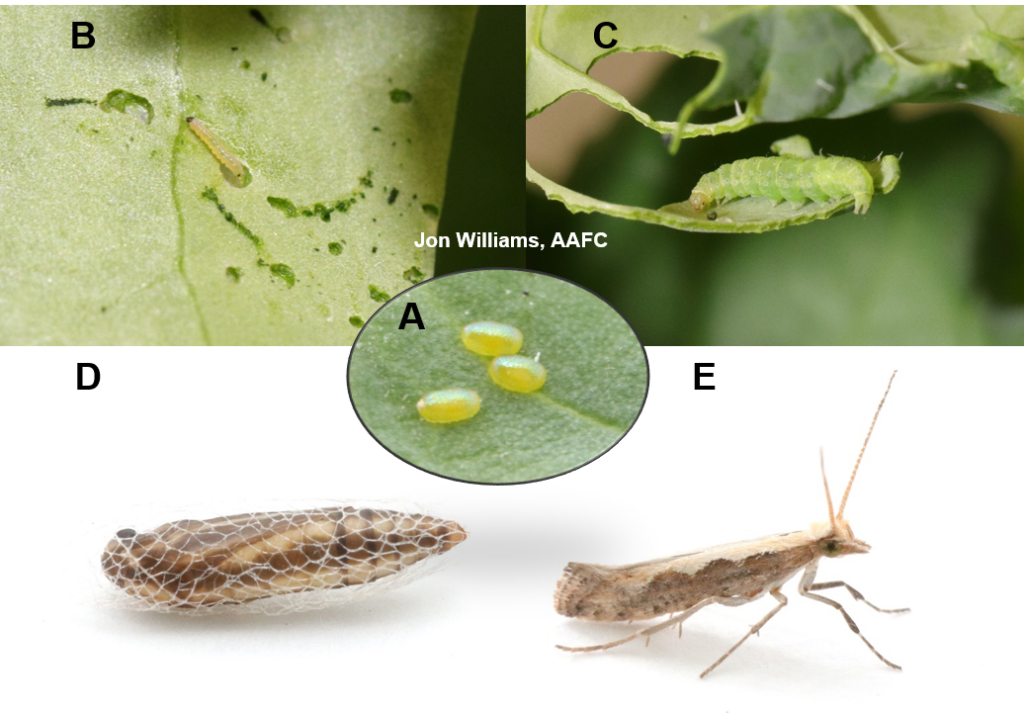
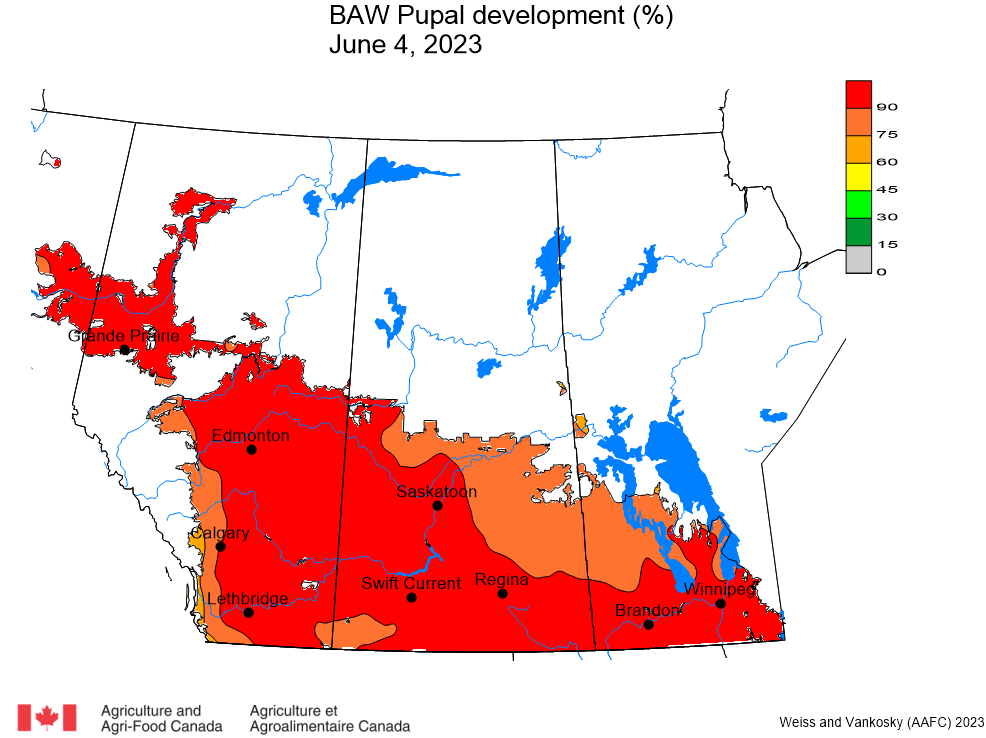
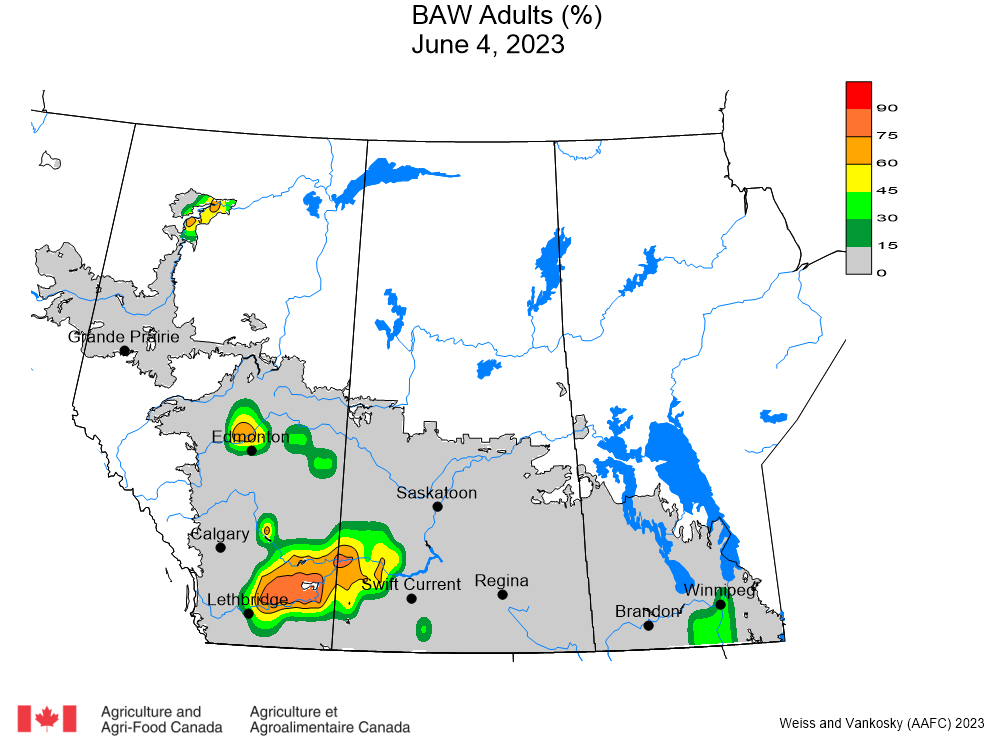
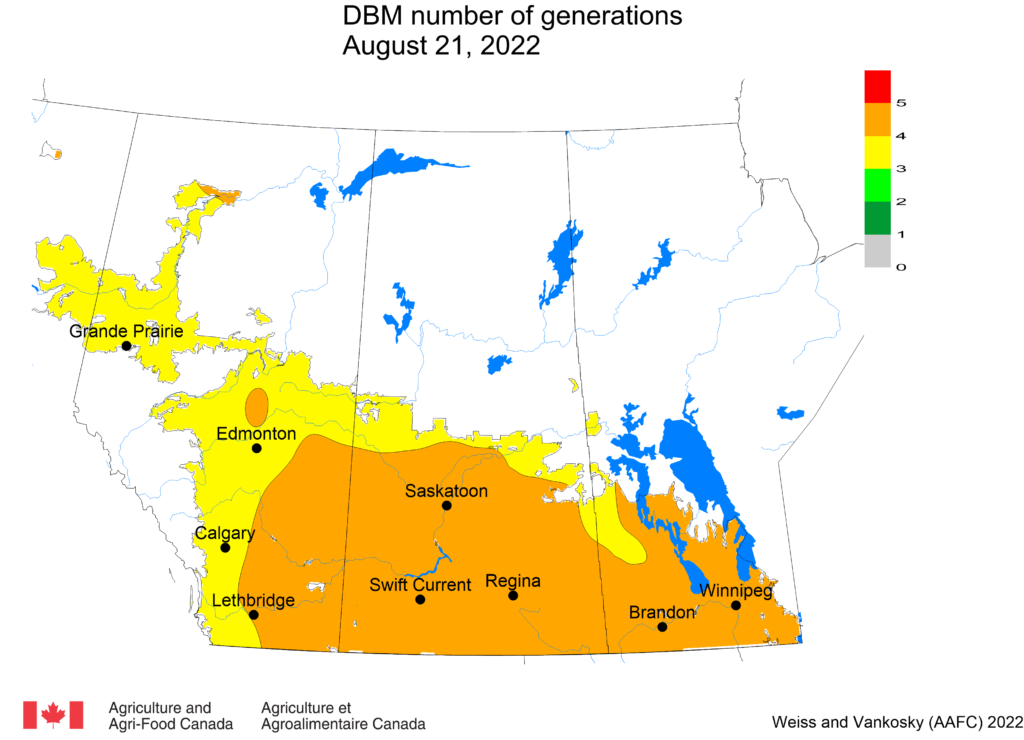
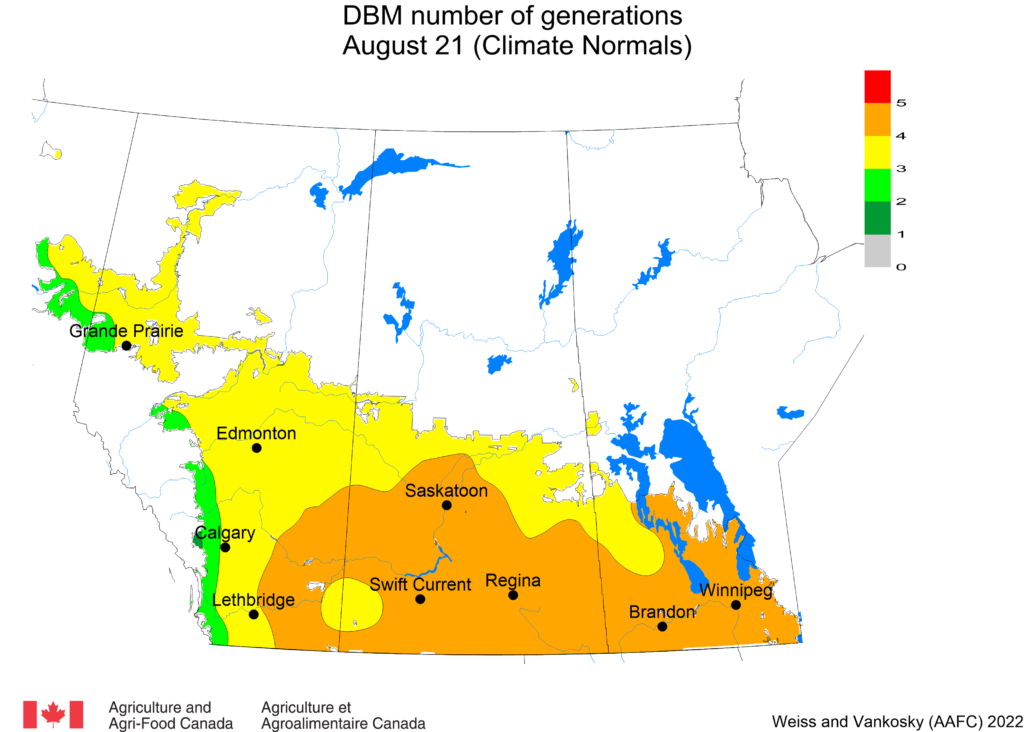
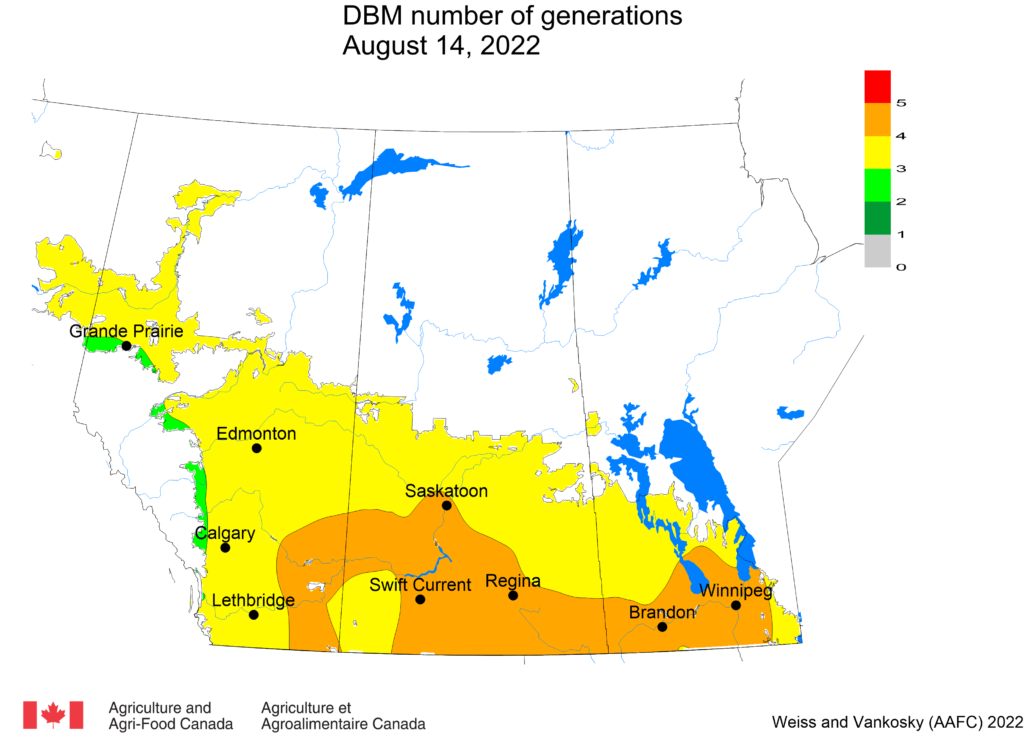
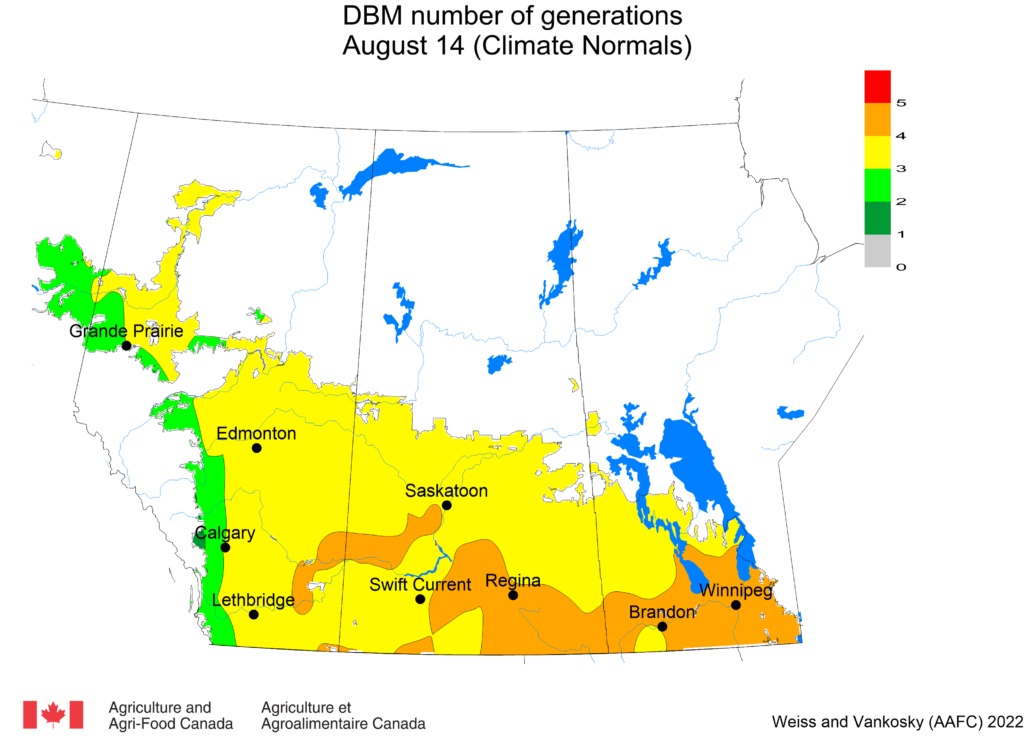
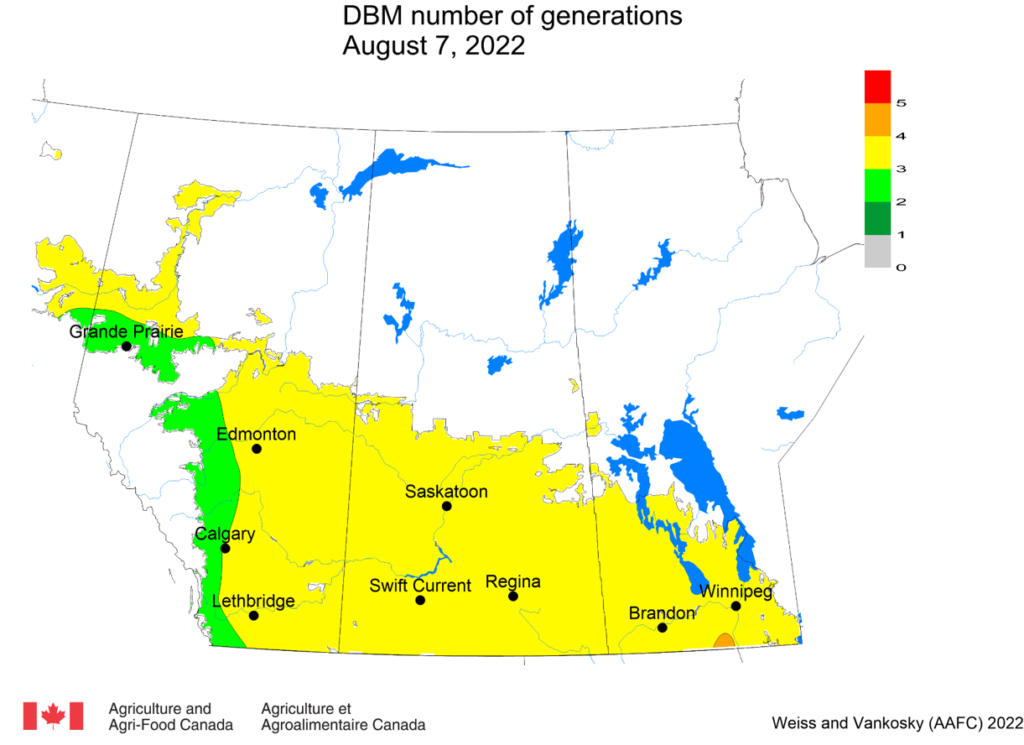
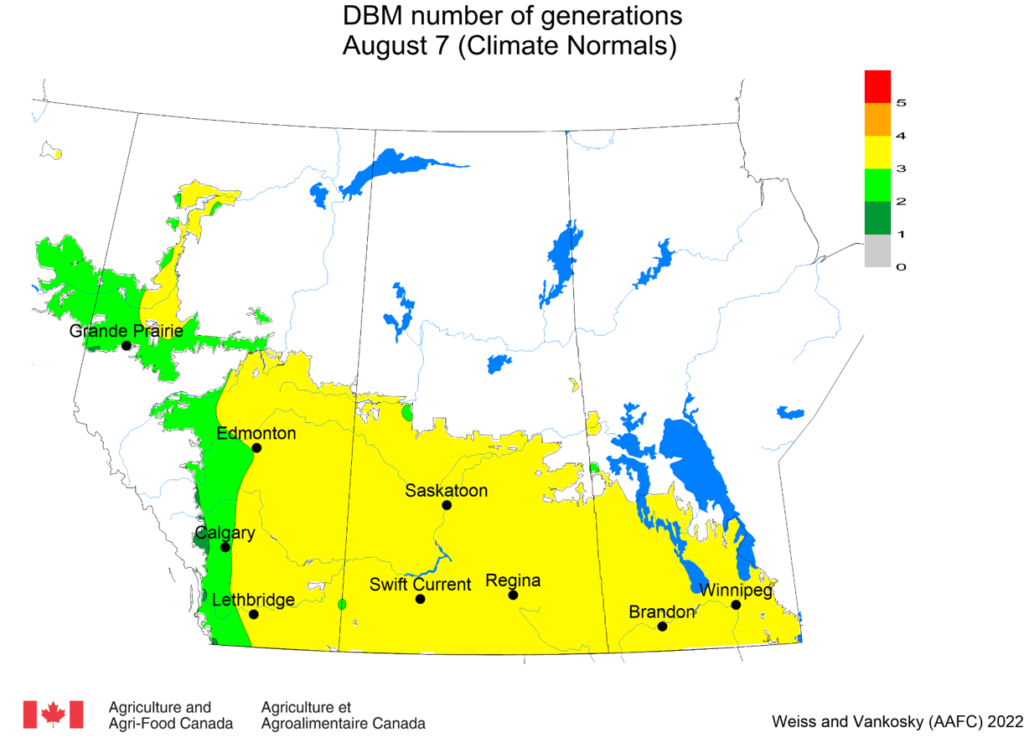
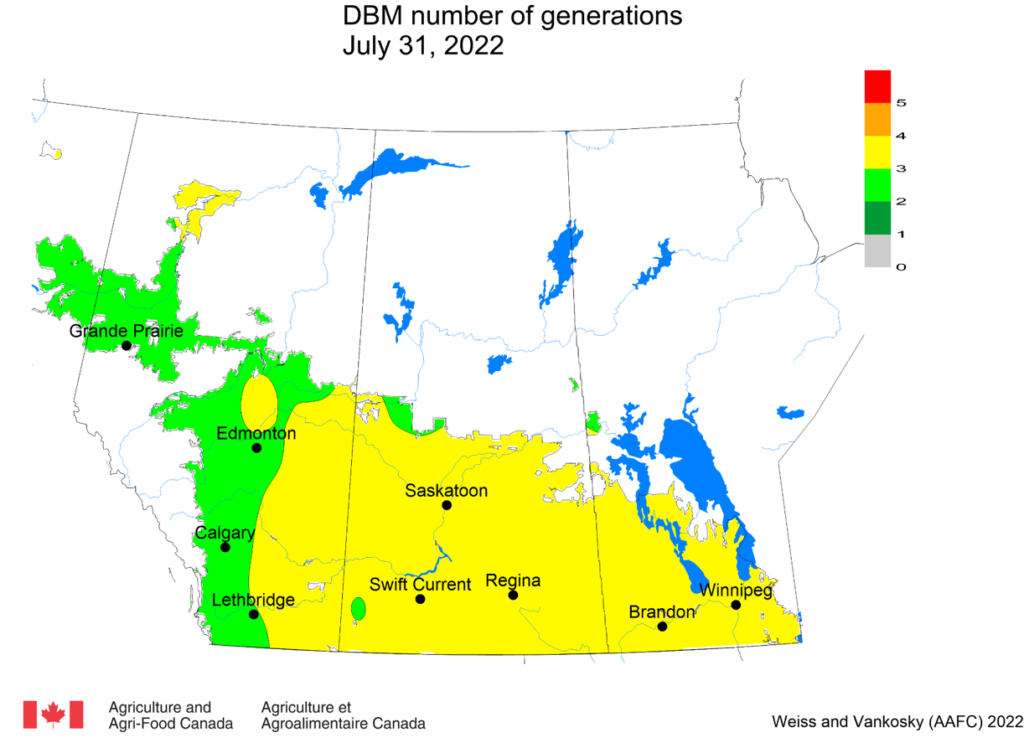
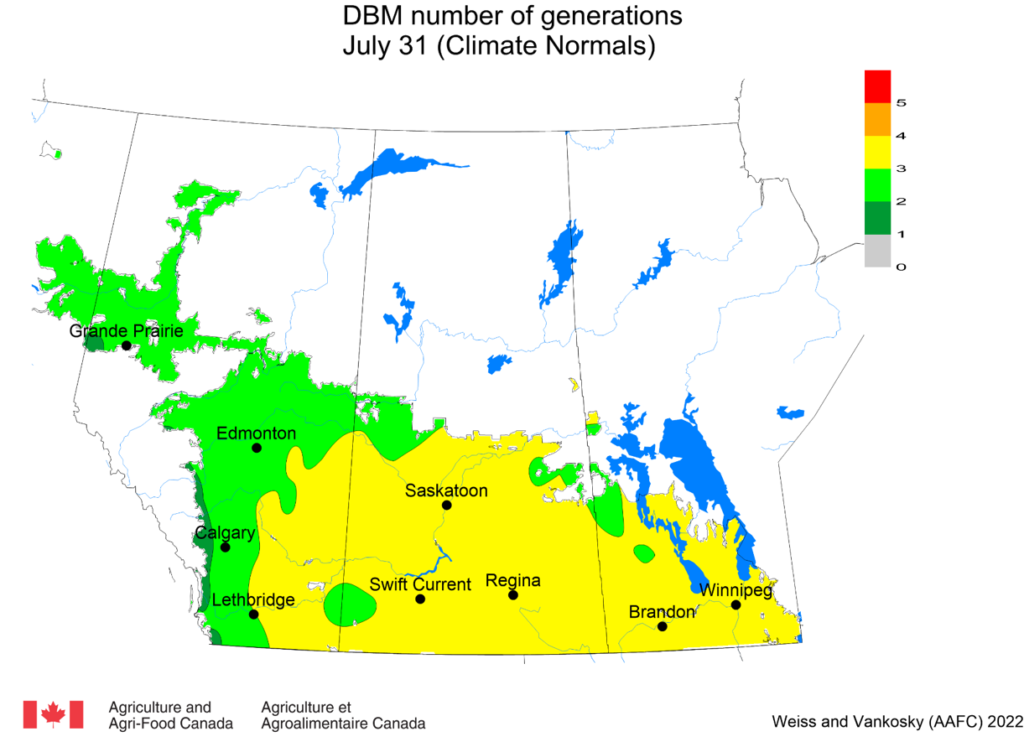
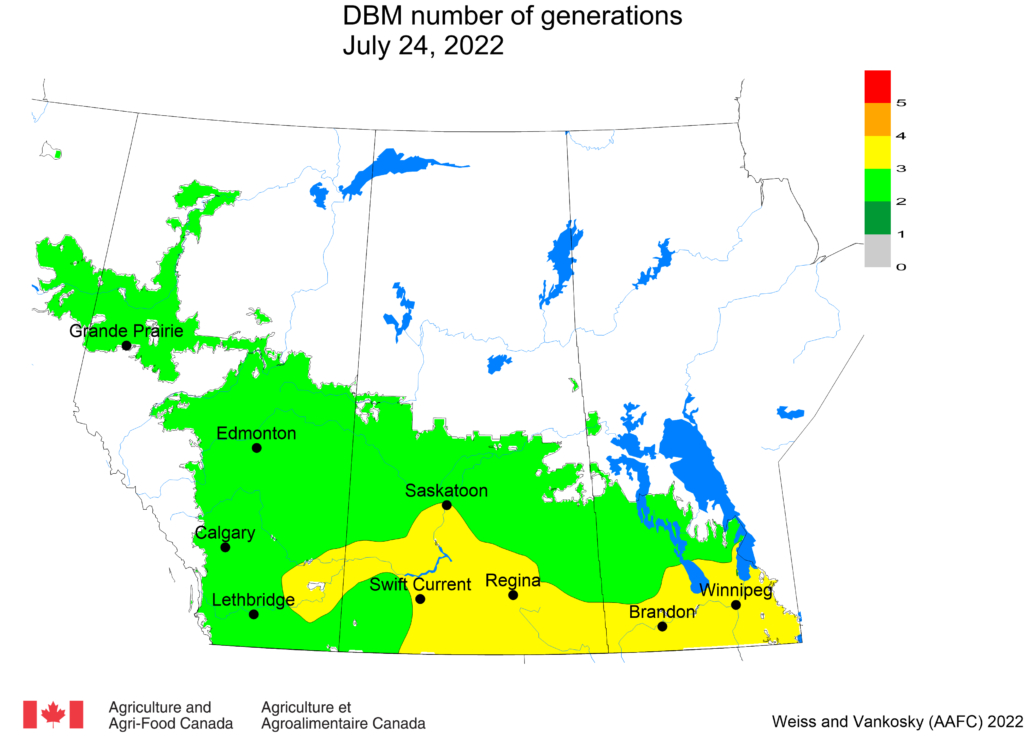
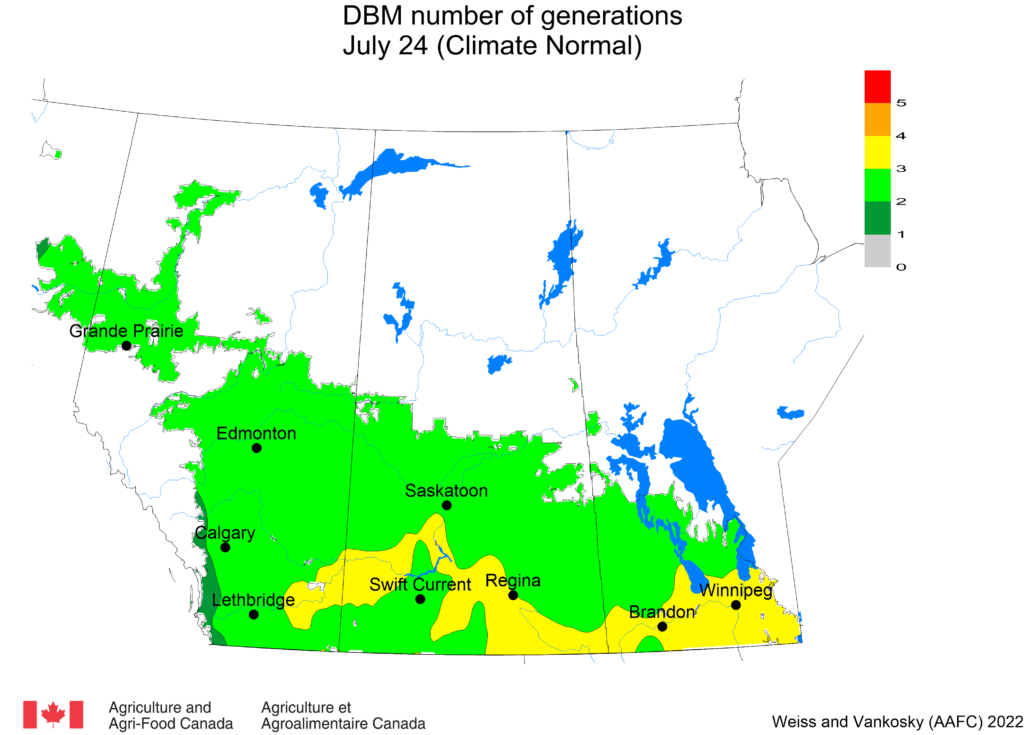
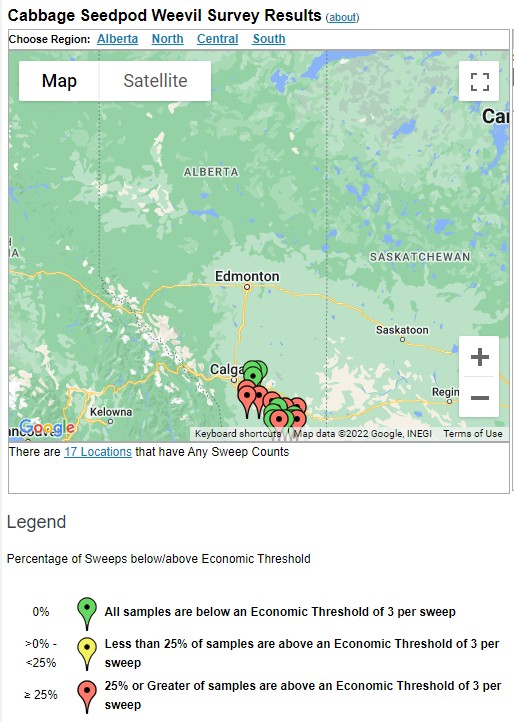
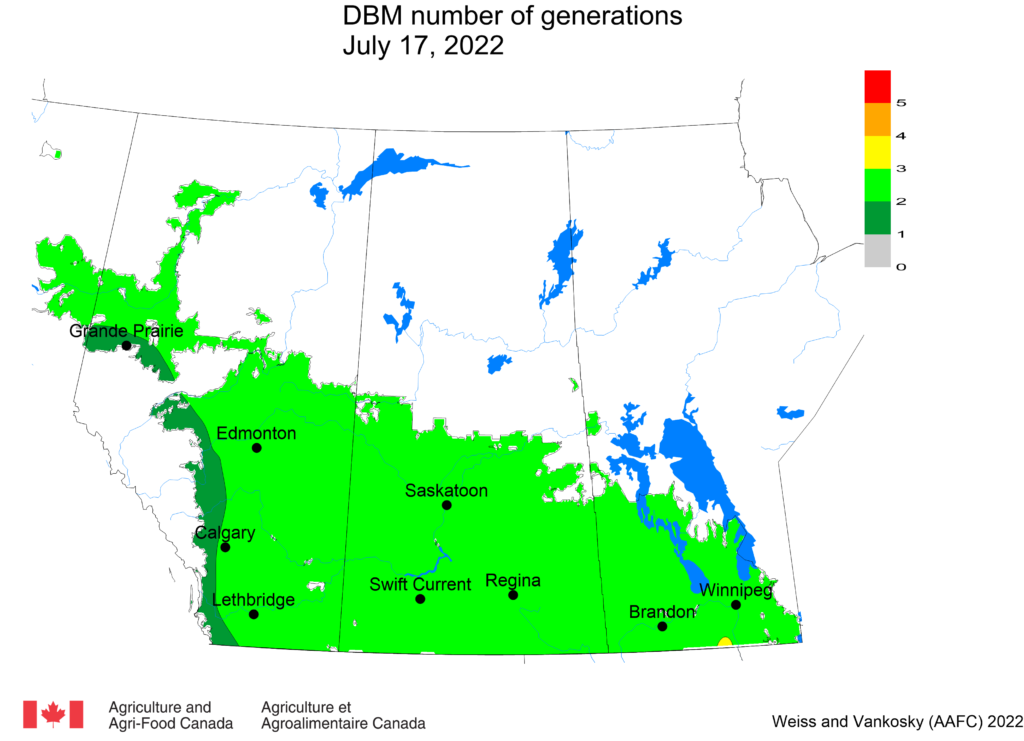
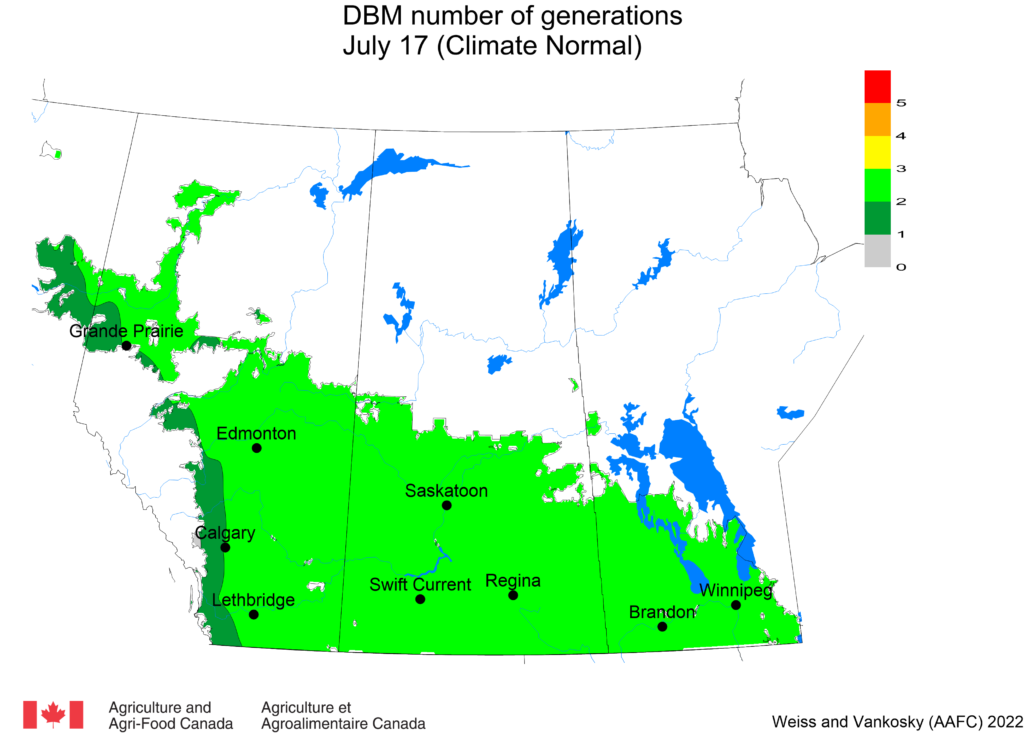
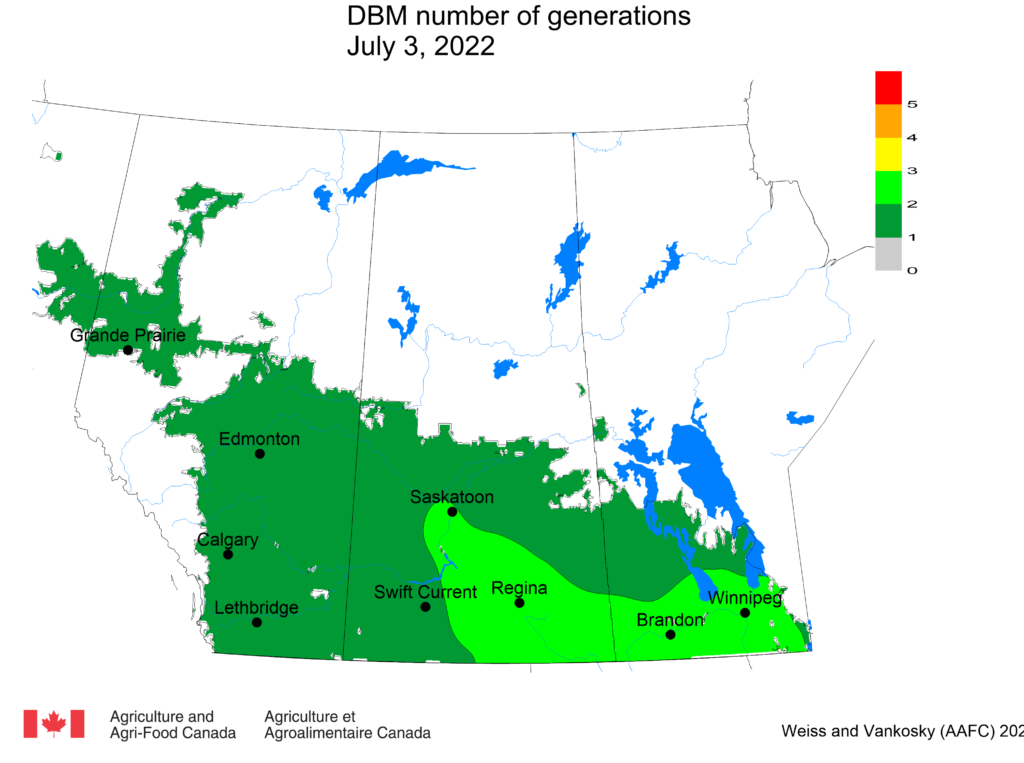
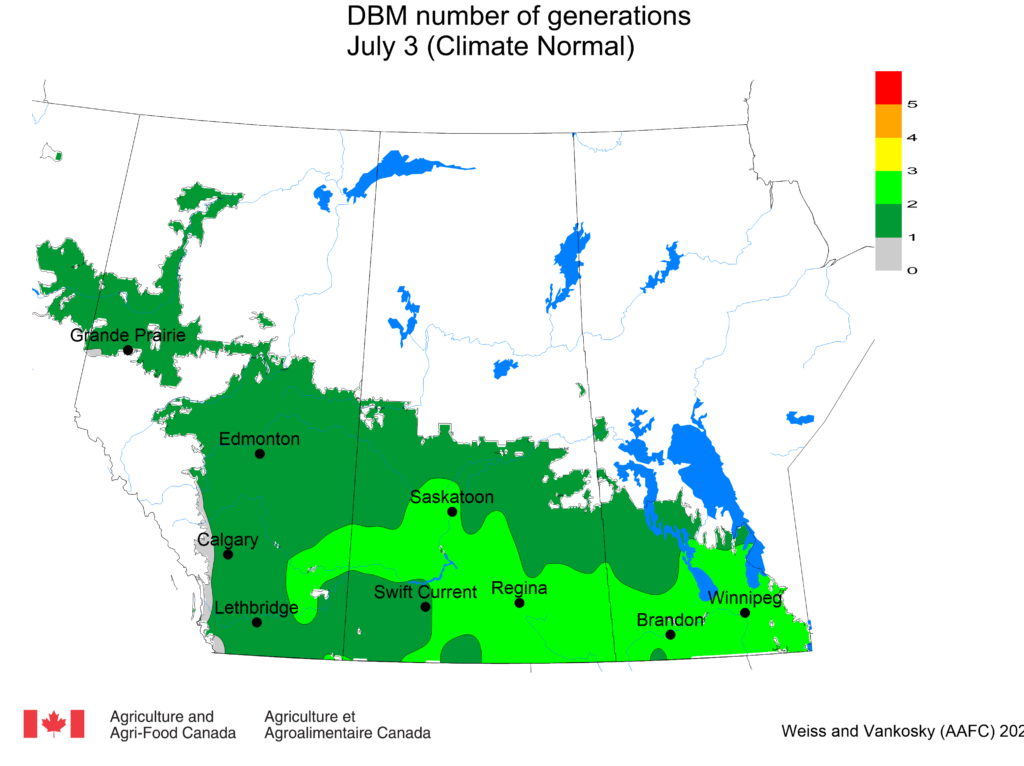
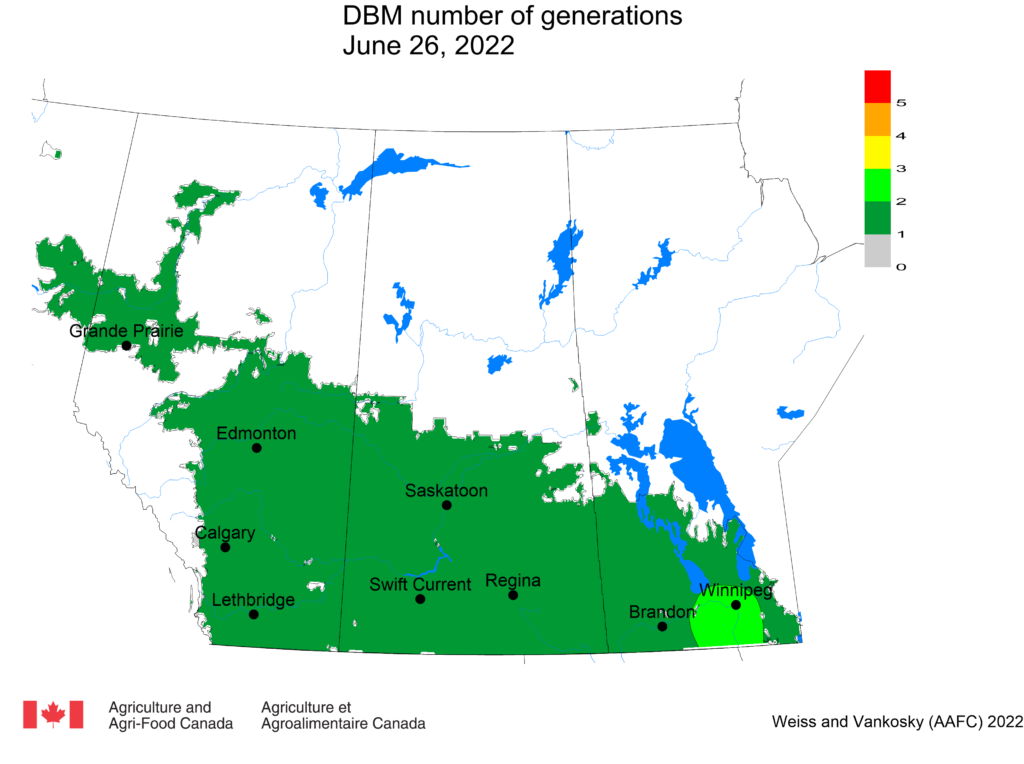
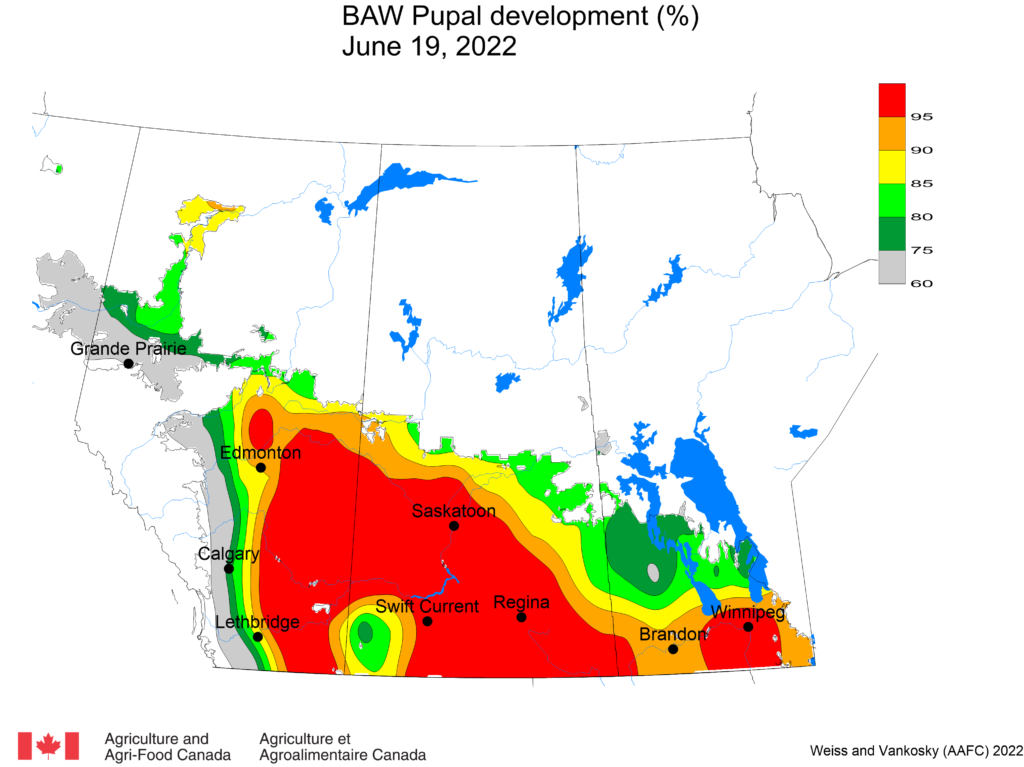
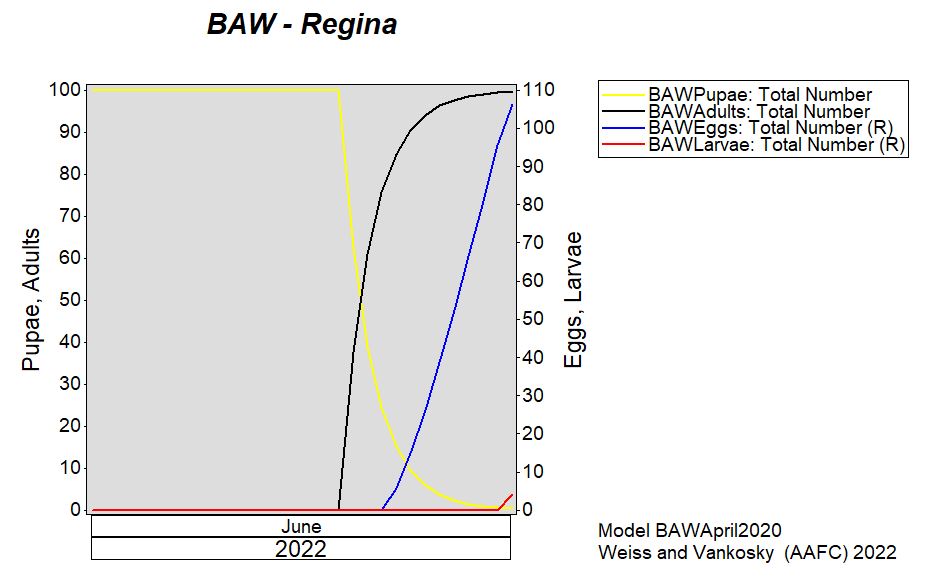
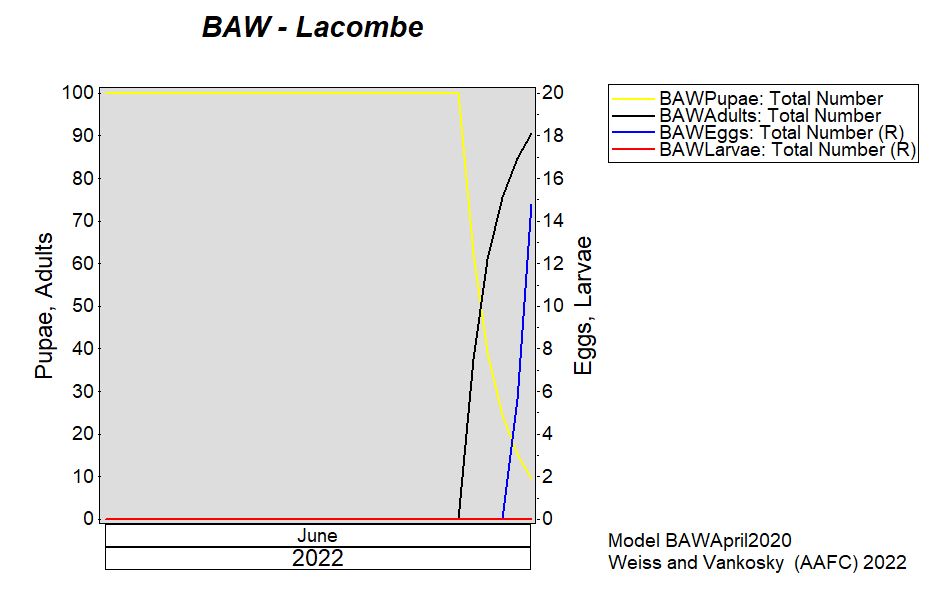
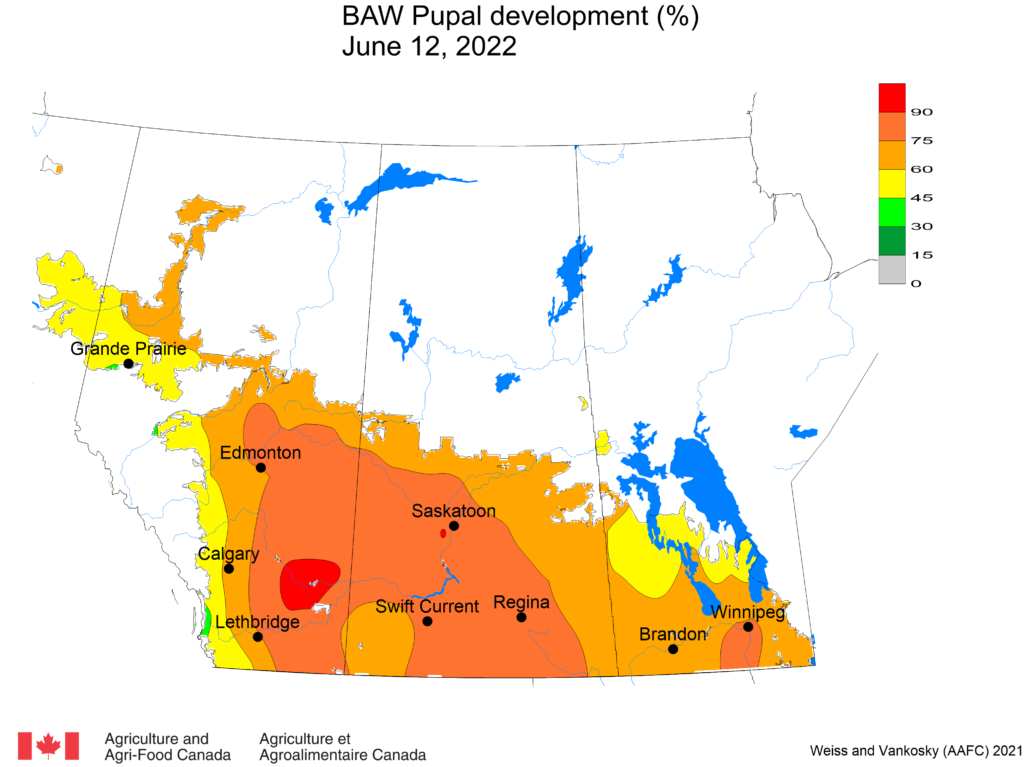
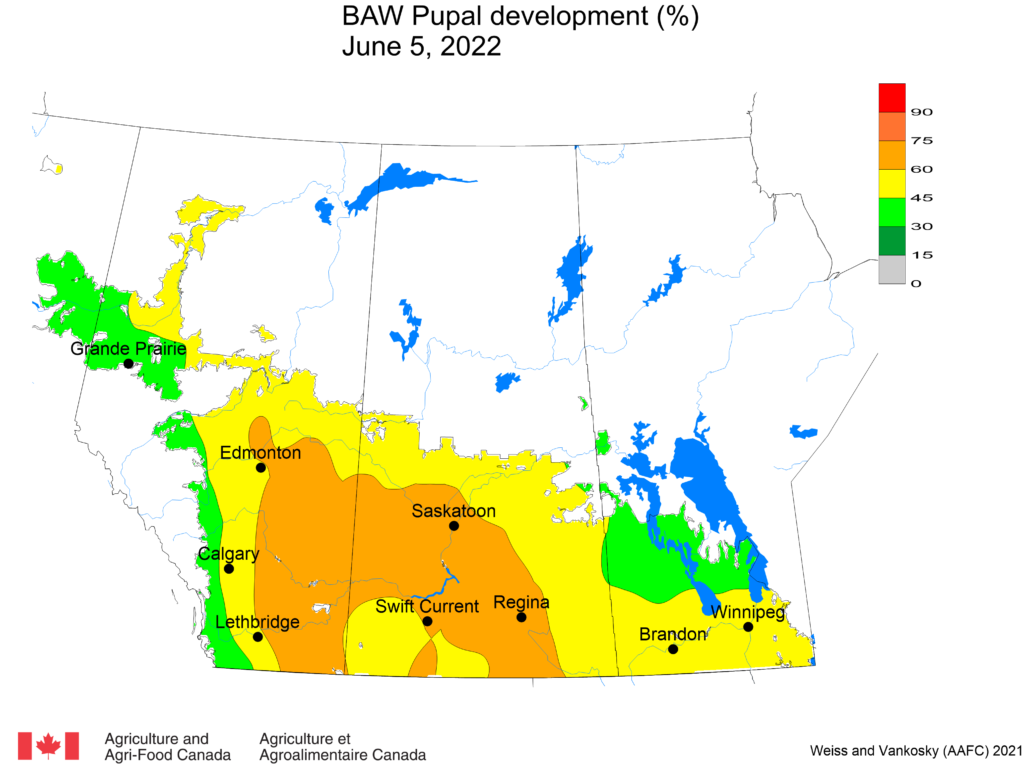
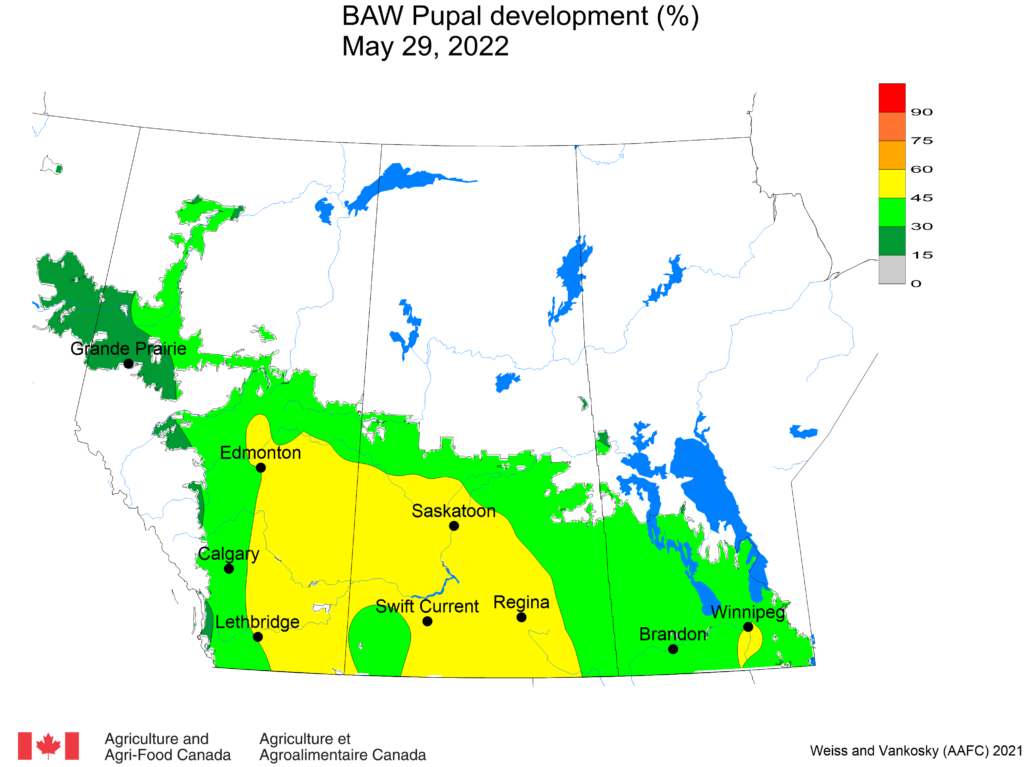
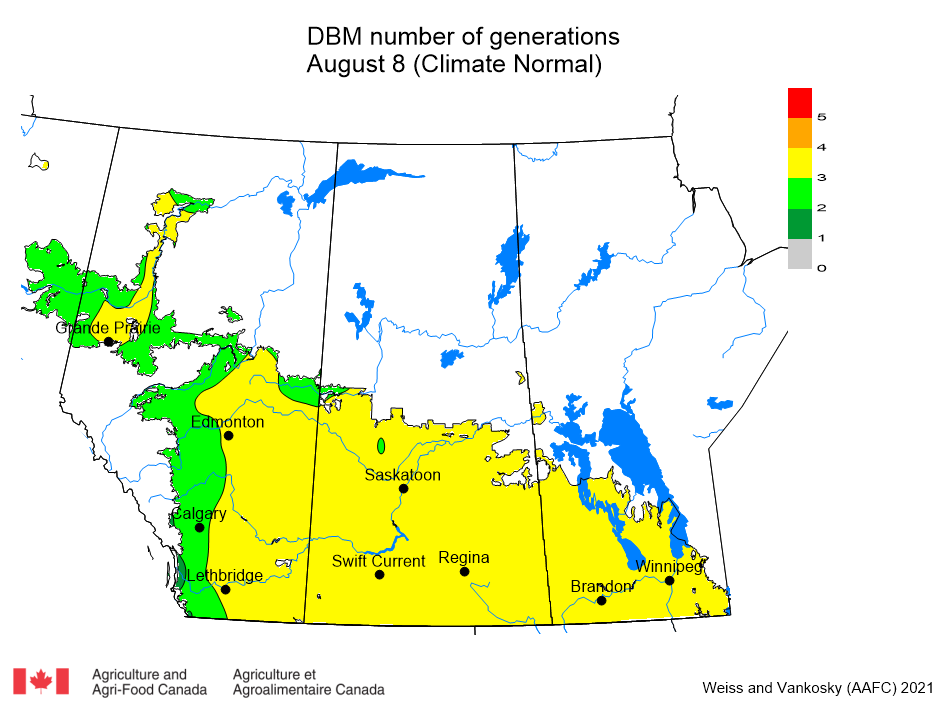
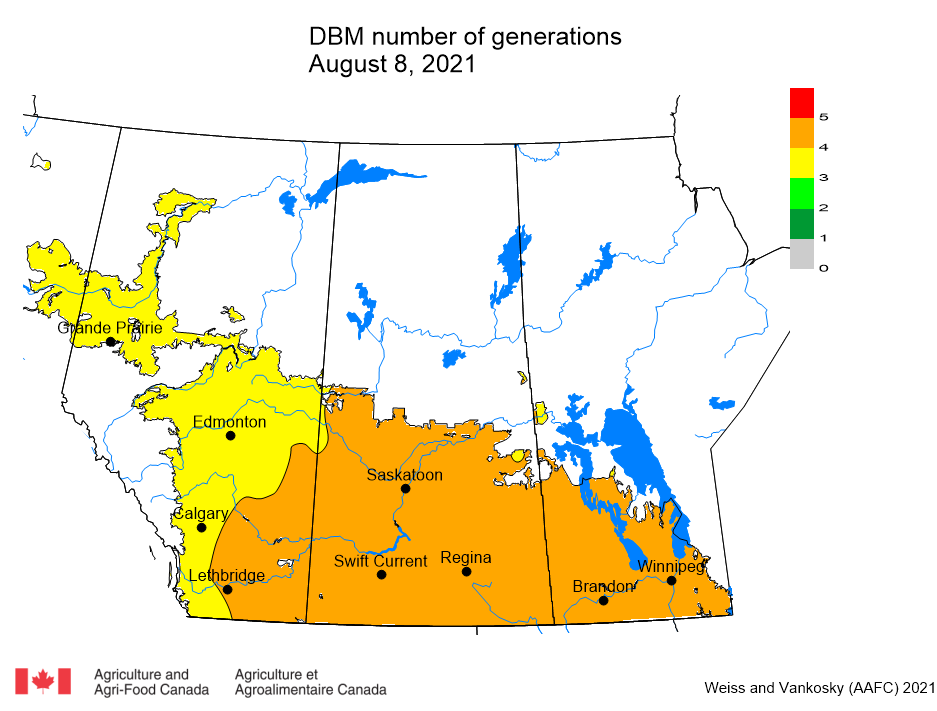
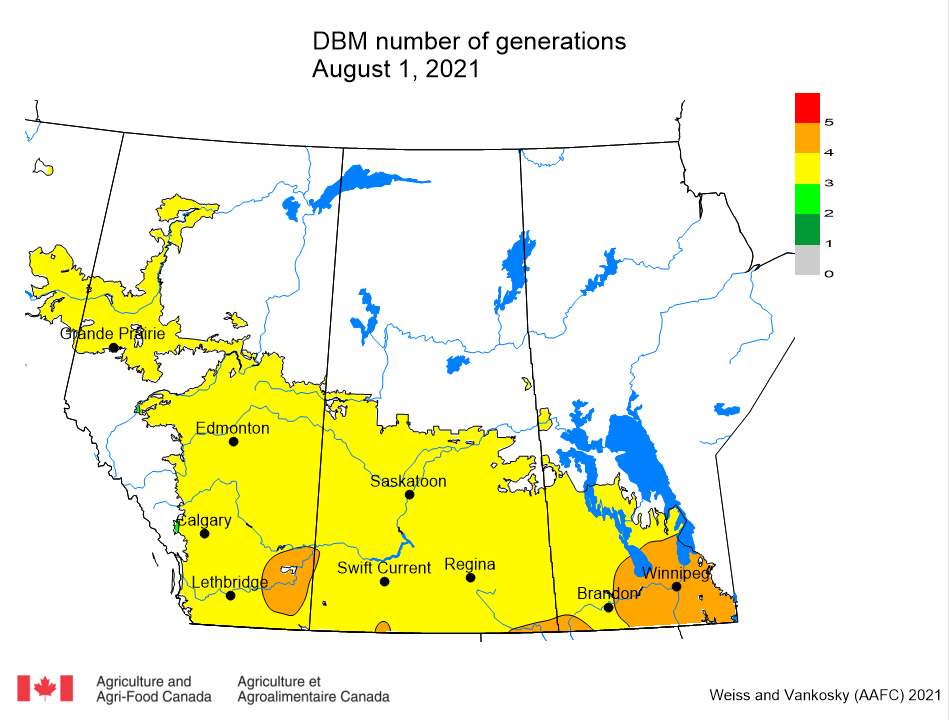
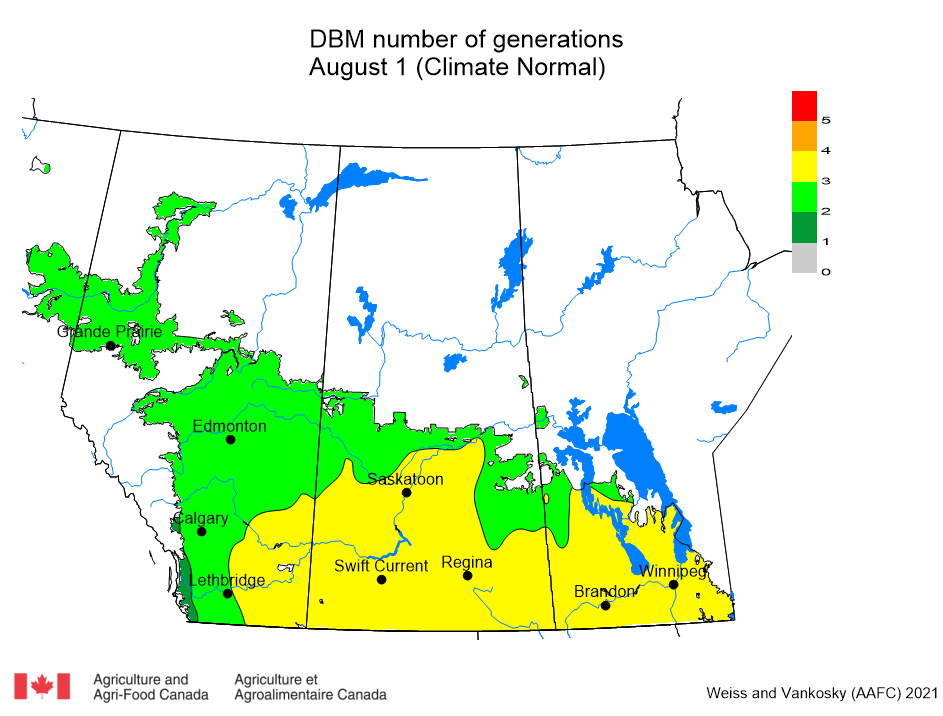
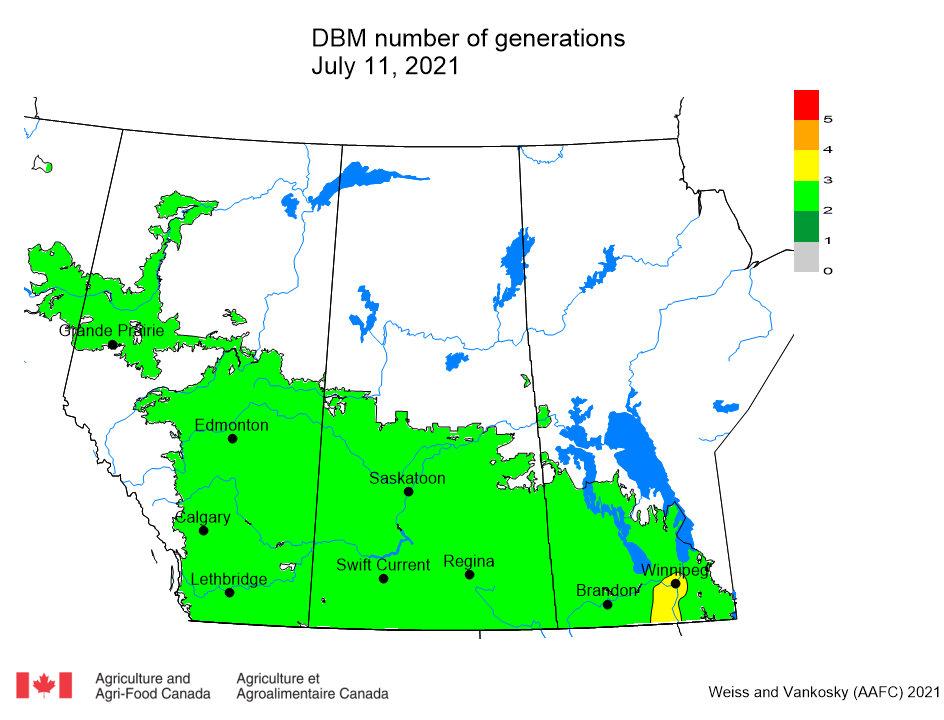
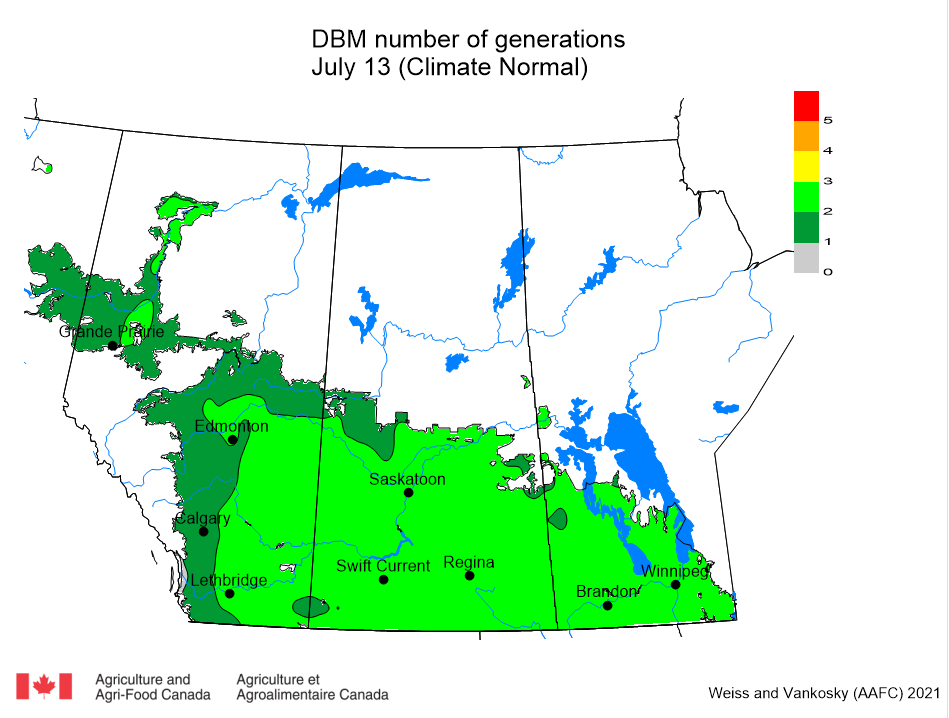
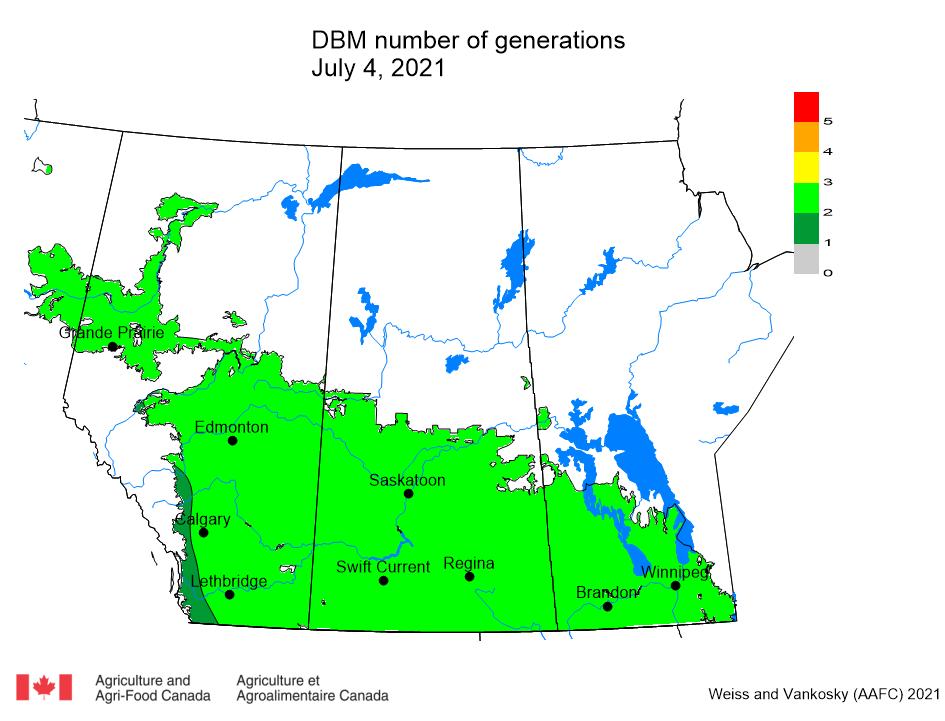
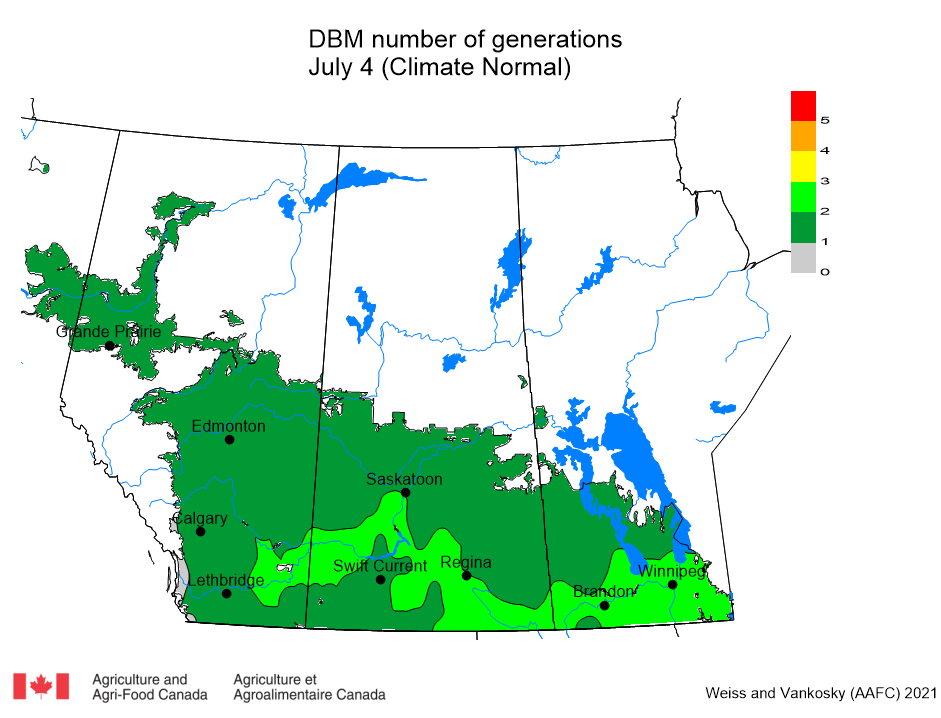
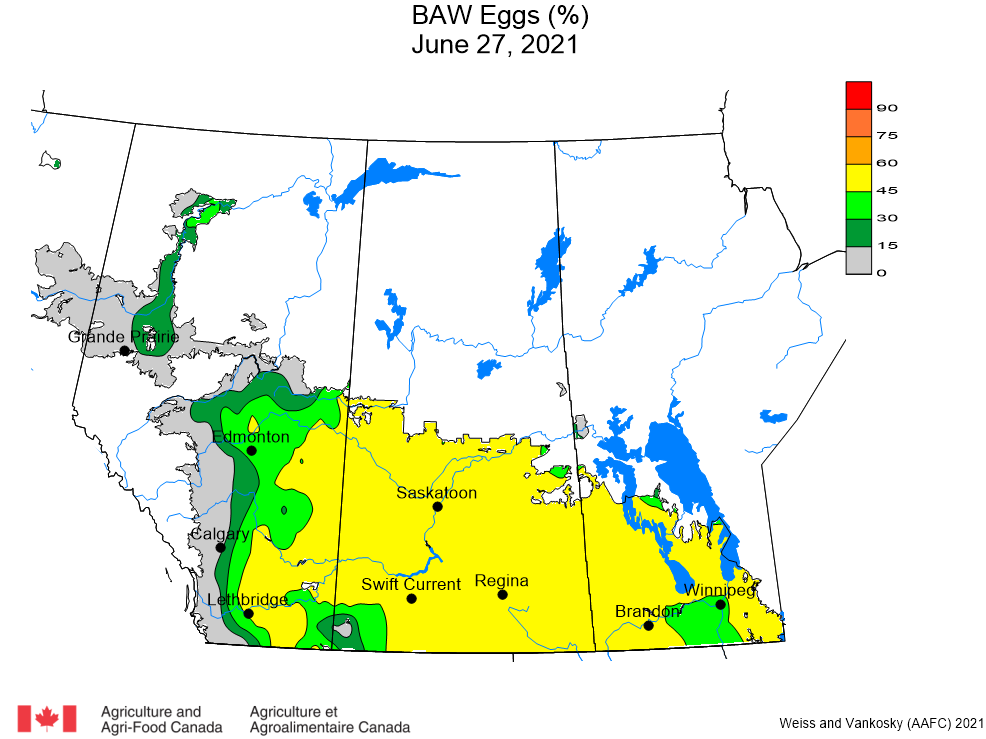
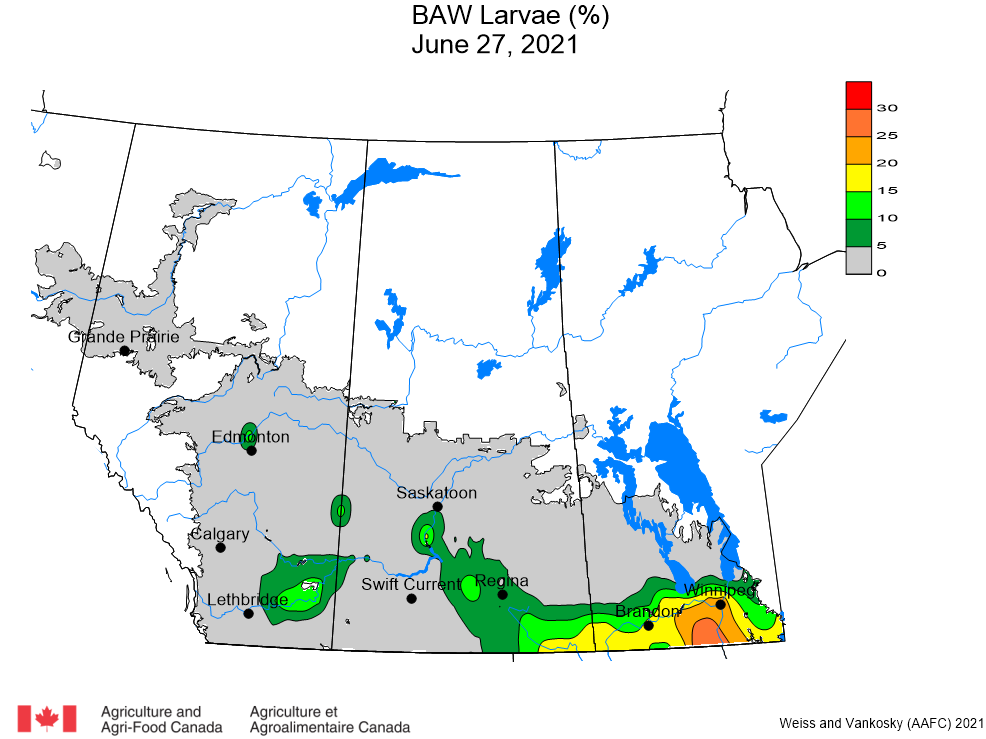
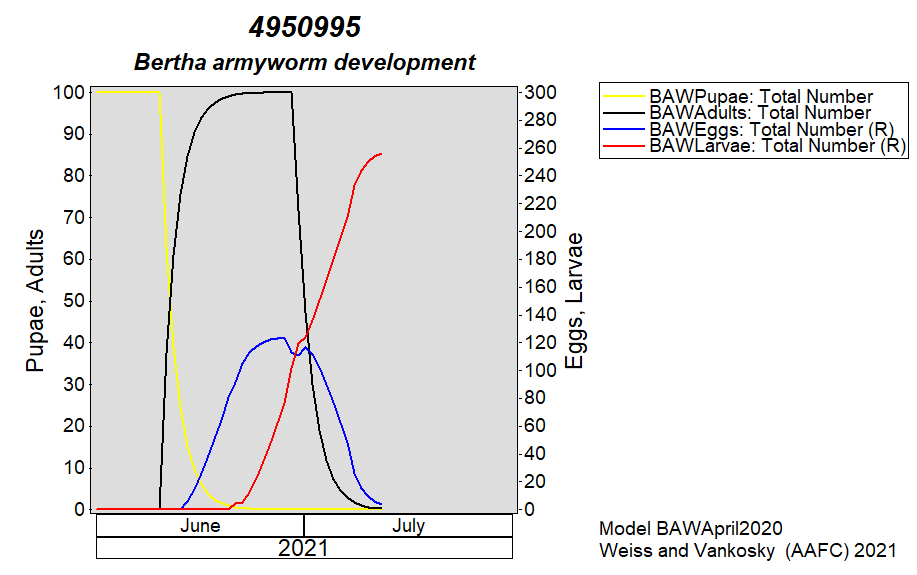
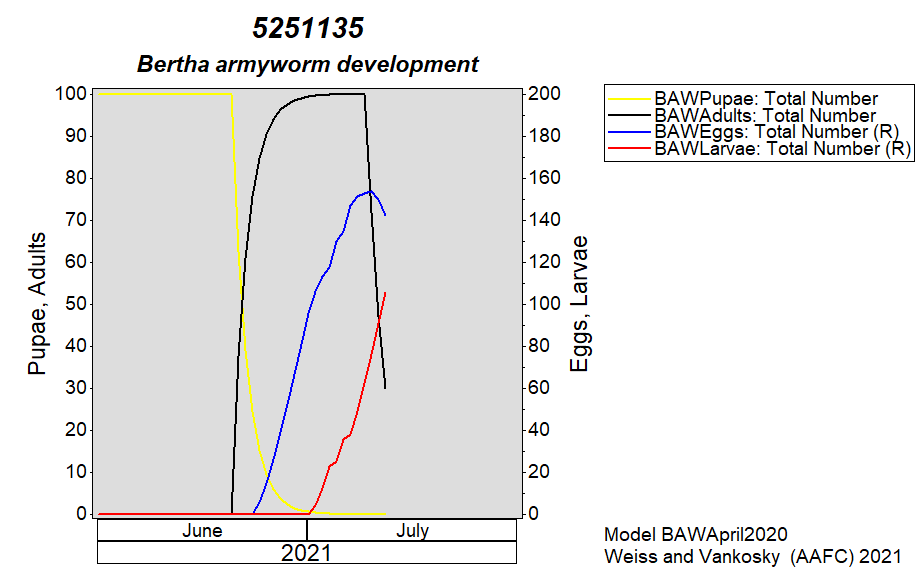
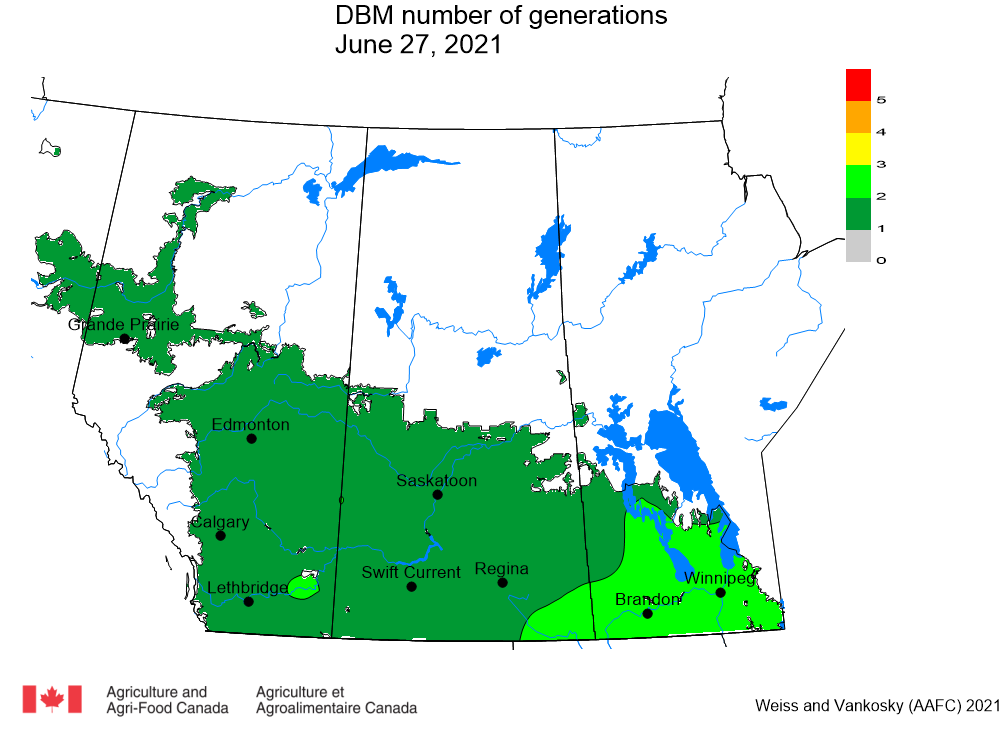
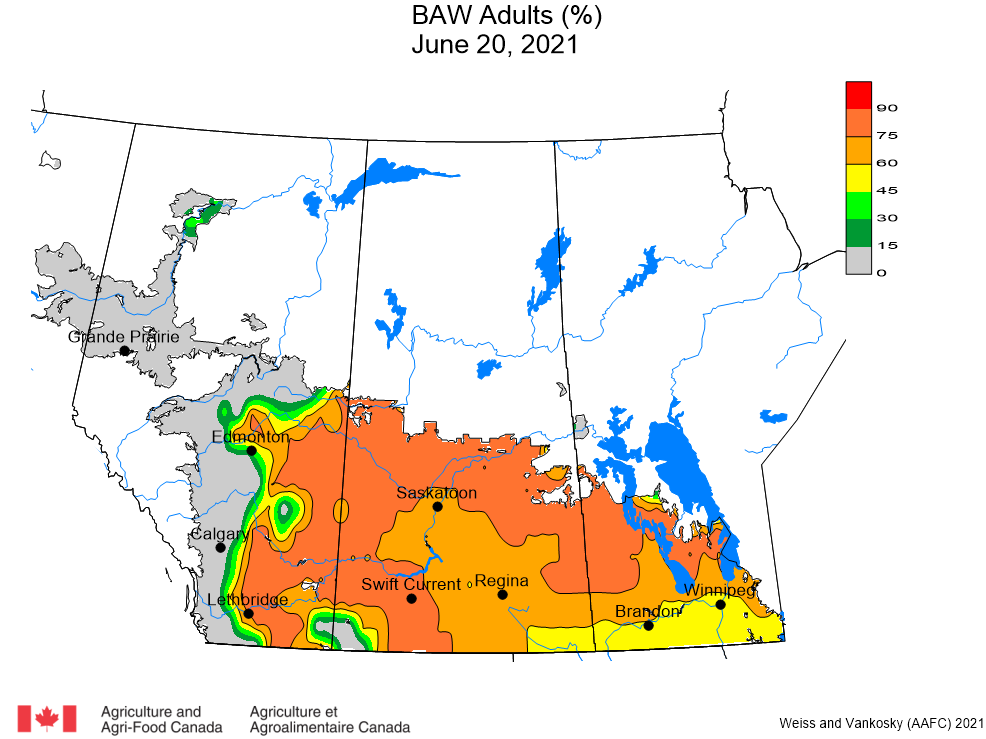
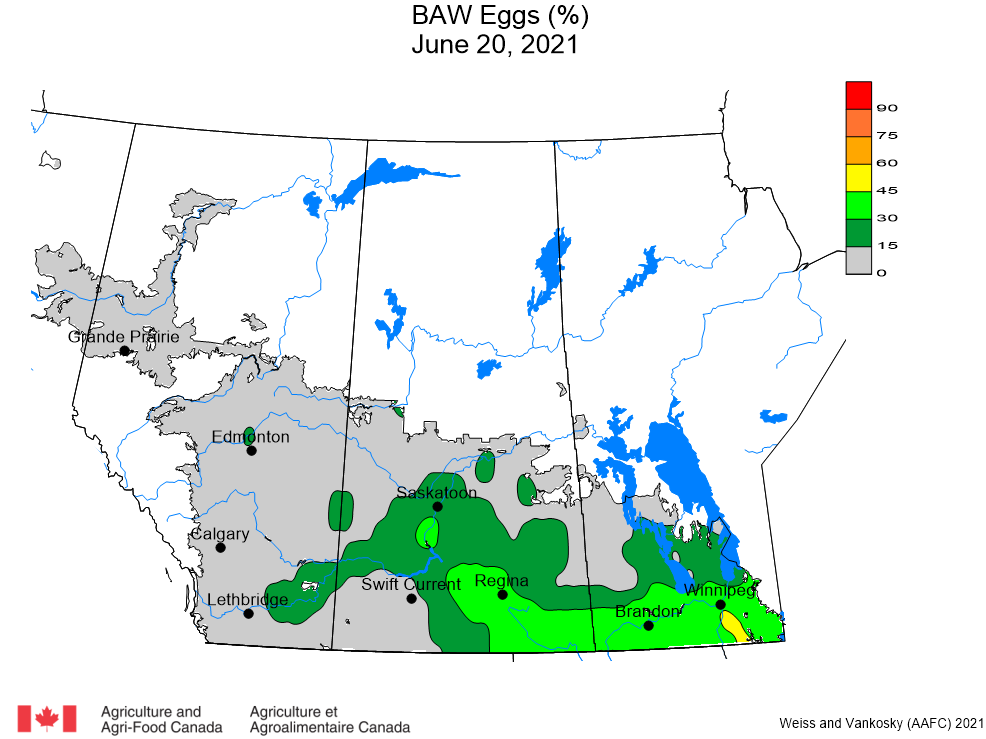
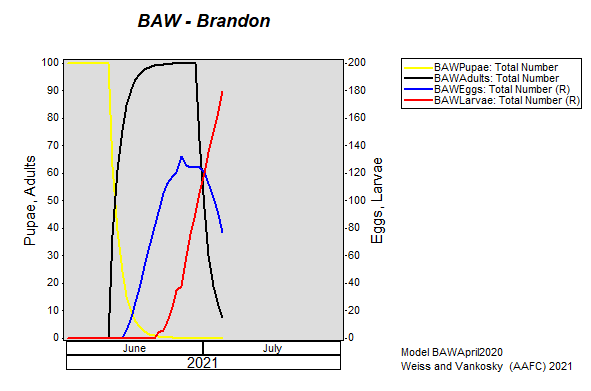
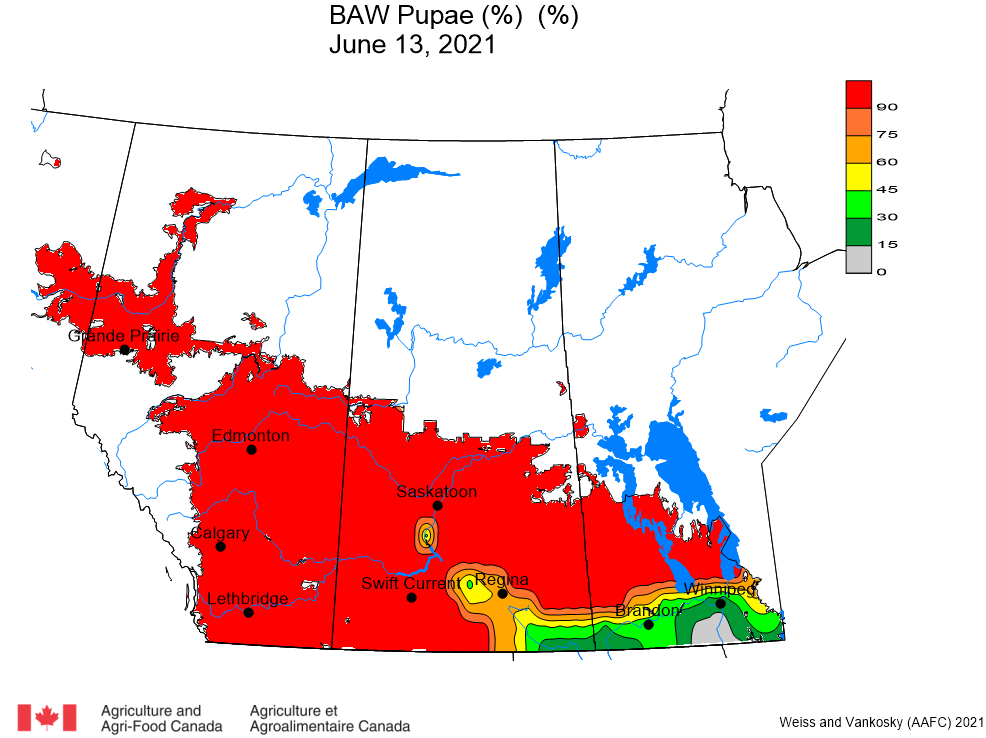
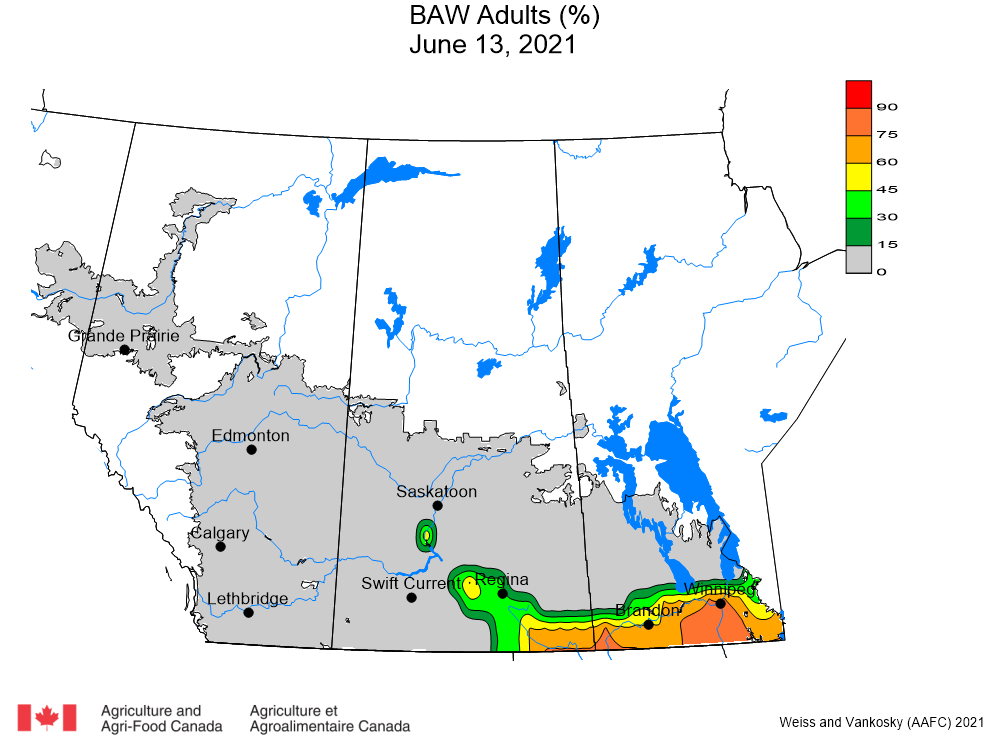
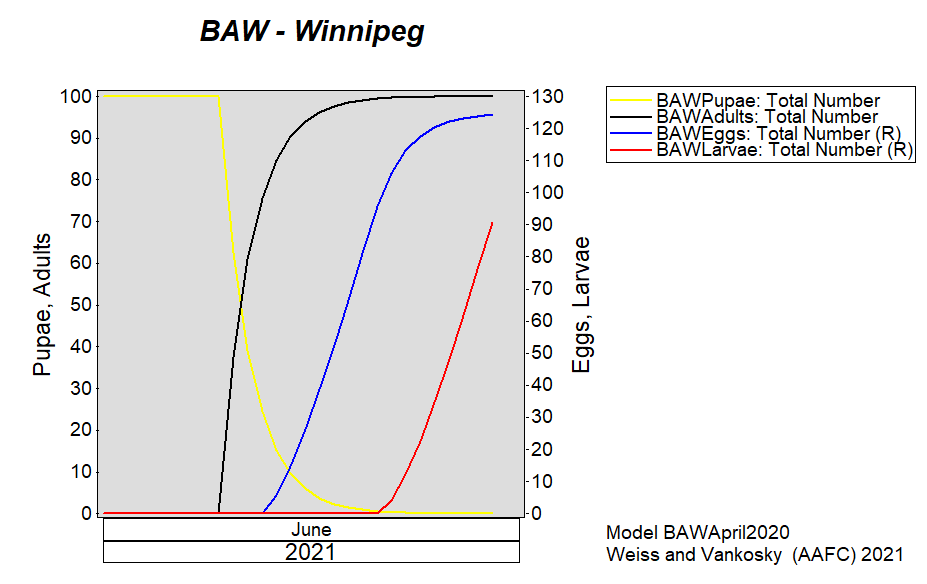
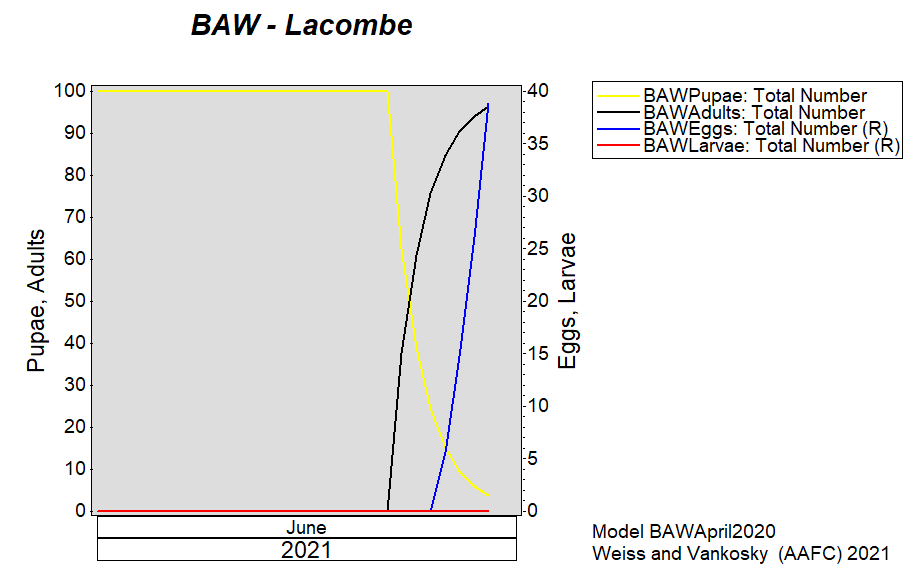
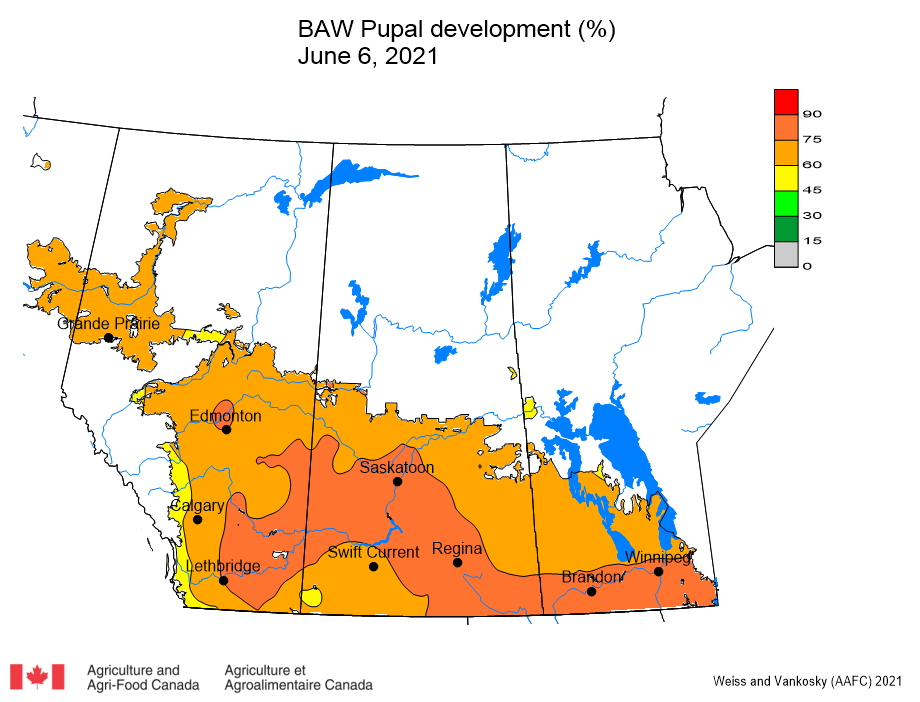
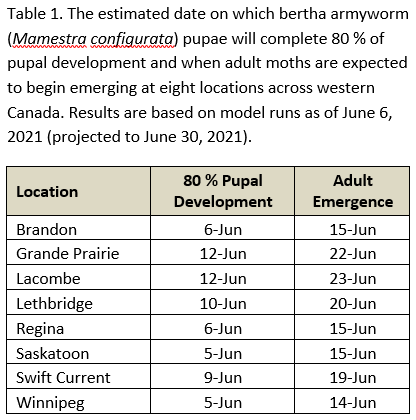
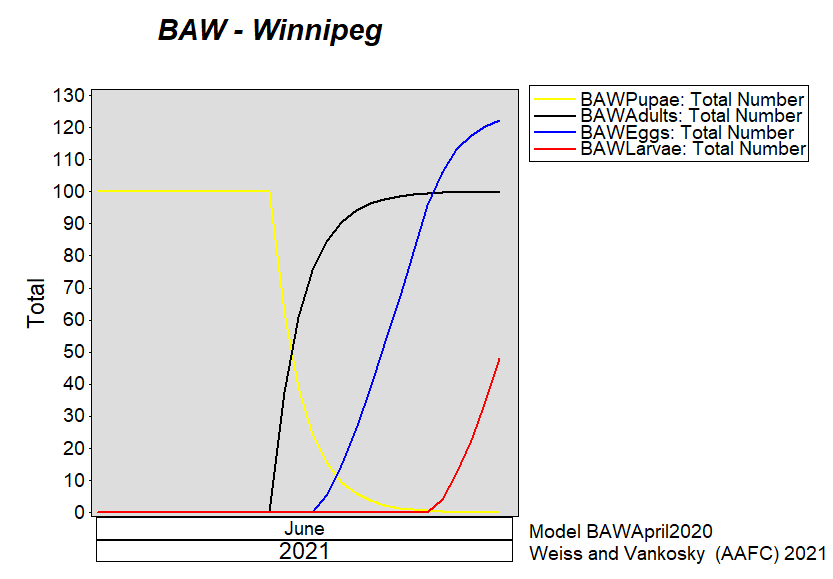
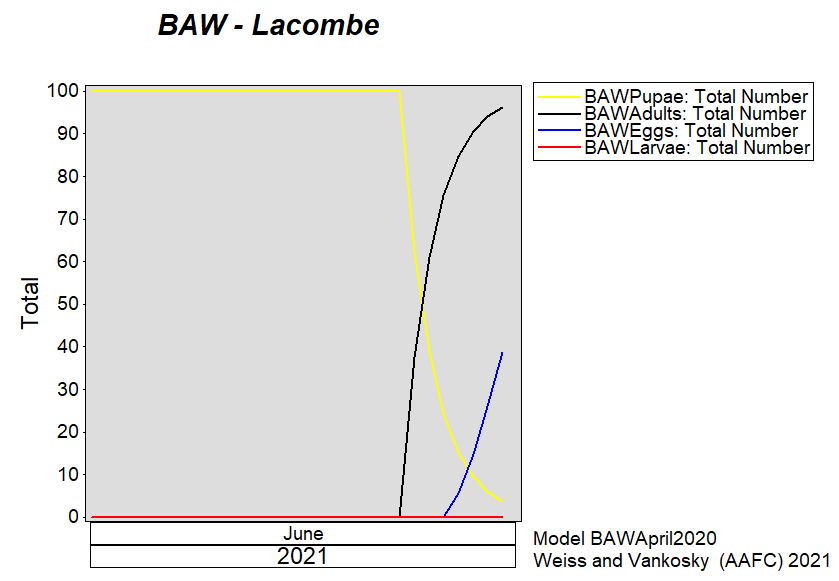
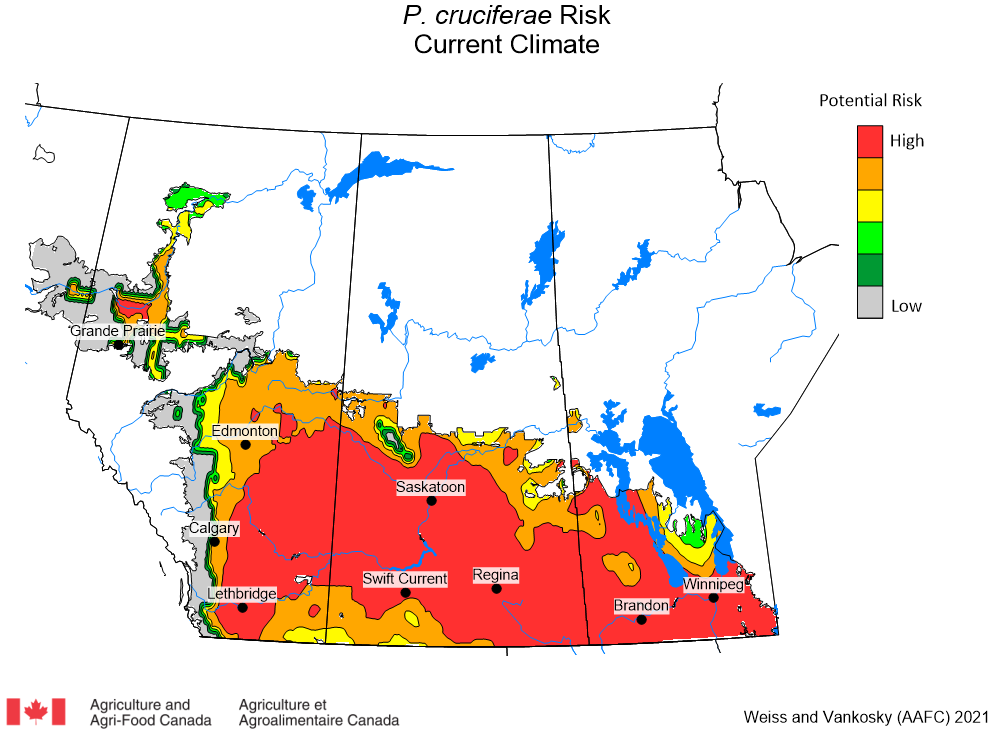
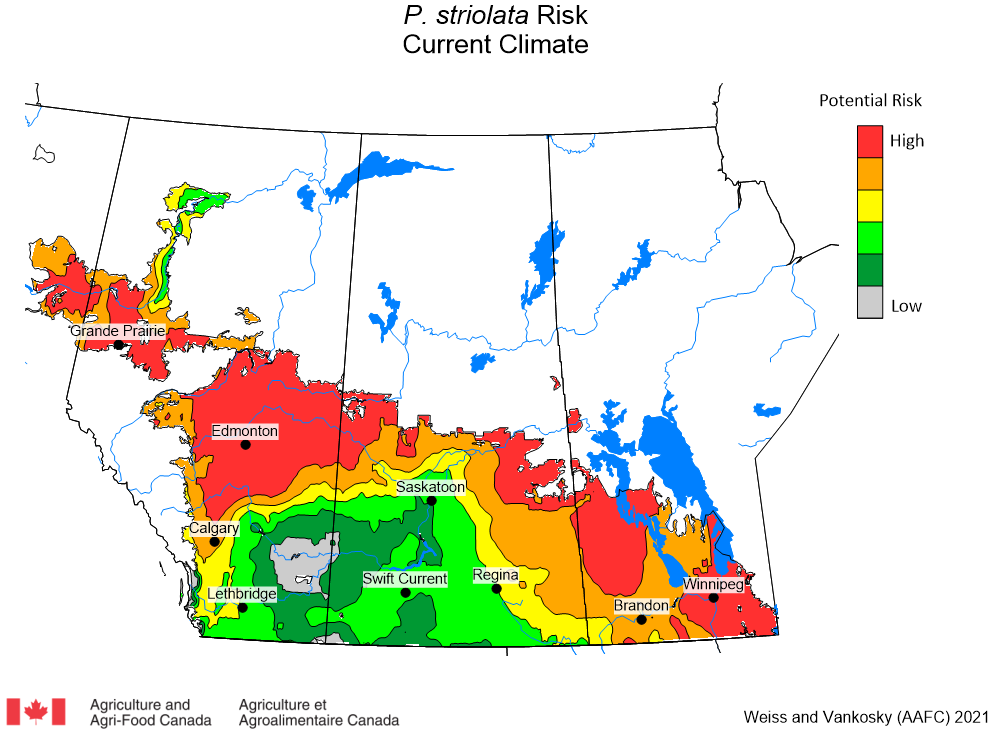
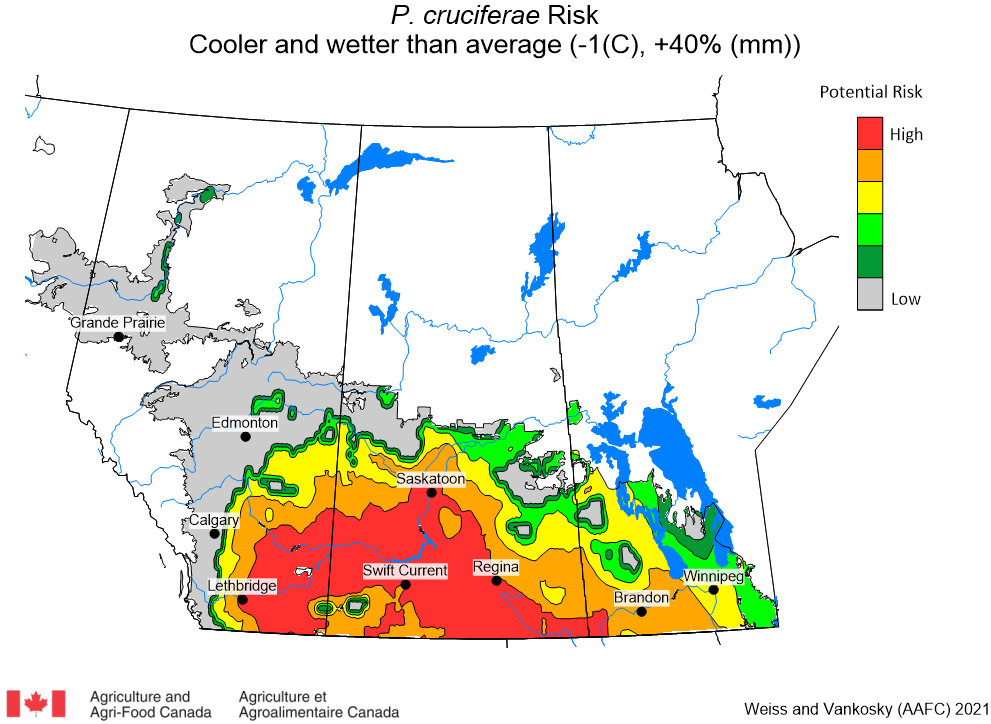
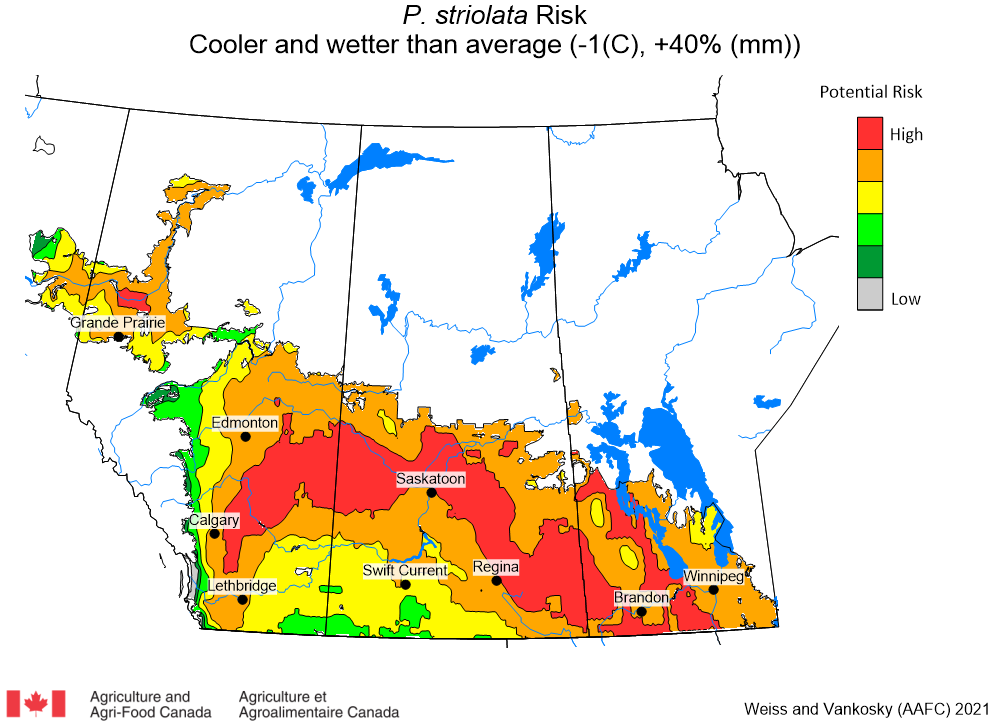
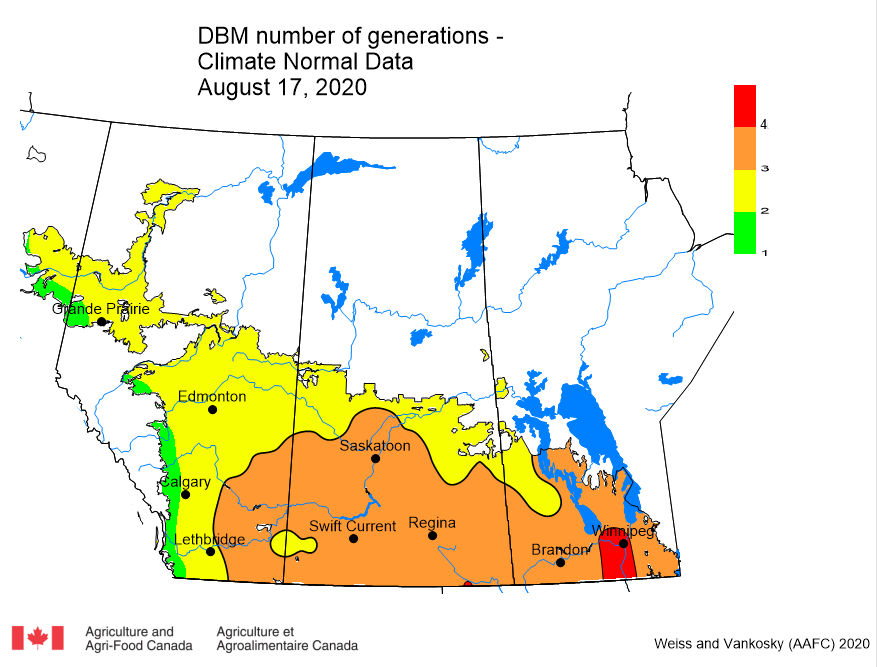
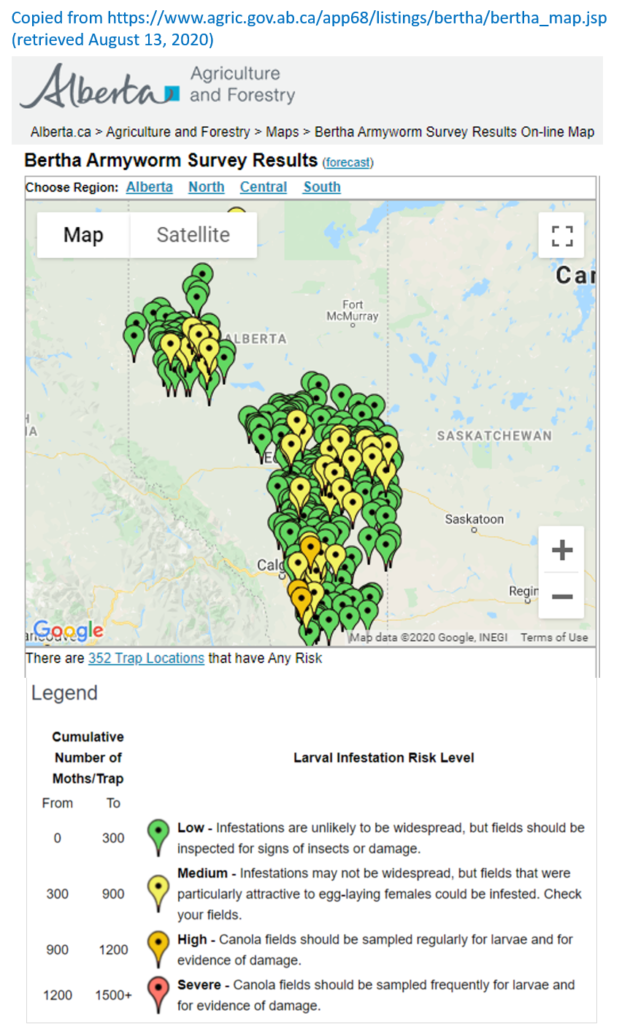
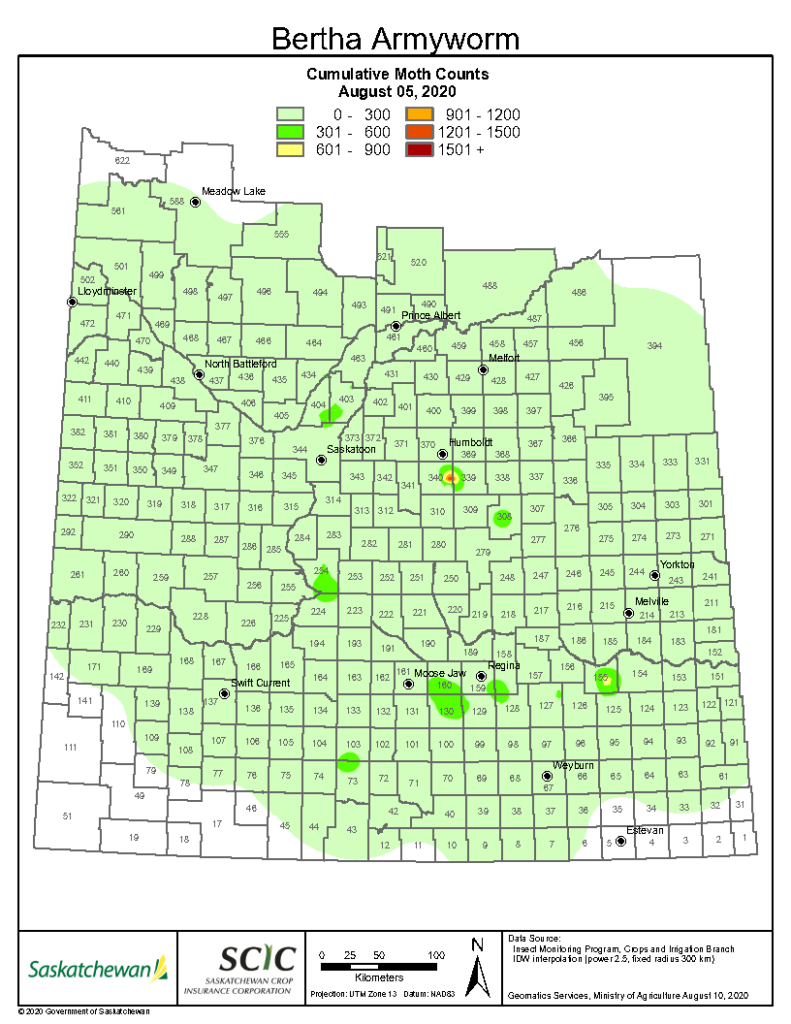
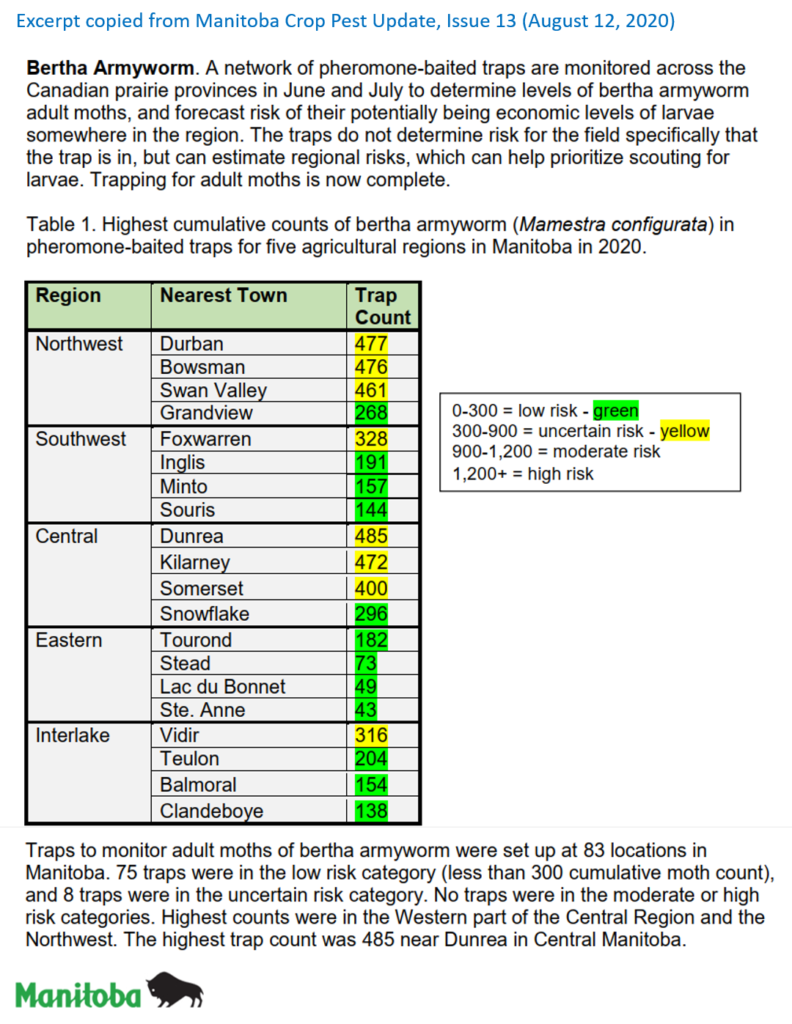
Aphid populations can quickly increase at this point in the season and particularly when growing conditions are warm and dry. Access the Provincial Insect Pest Report for Wk12 to remain alert to areas and crops suffering from aphid pest pressure.

Biological and monitoring information (including tips for scouting and economic thresholds) related to aphids in field crops is posted by:
• Manitoba Agriculture (aphids on cereals, aphids on flax, soybean aphid, aphids on peas)
• Saskatchewan Pulse Growers (aphids in pulse crops or access the PDF copy)
• Saskatchewan Flax Development Commission
• Manitoba Pulse and Soybean Growers (soybean aphids: identification, scouting and management or access the PDF copy)
• The Canola Council of Canada’s “Canola Encyclopedia” section on aphids
• or check your provincial commodity group’s insect pest webpages for more detailed information.
Alternatively, several aphid pest species are described in the “Field Crop and Forage Pests and their Natural Enemies in Western Canada: Identification and management field guide” (2018) and is accessible as a free downloadable PDF in either English or French on our Field Guides page. PDF copies of the individual pages have been linked below to access quickly:
• Corn leaf aphid or Rhopalosiphum maidis (Fitch)
• English grain aphid or Sitobion (Macrosiphum) avenae (Fabricius)
• Oat-birdcherry aphid or Rhopalosiphum padi (Linnaeus)
• Pea aphid or Acyrthosiphon pisum (Harris)
• Potato aphid or Macrosiphum euphorbiae (Thomas)
• Soybean aphid or Aphis glycines (Matsumura)
• Turnip aphid or Lipaphis erysimi (Kaltenbach)
• Sugar beet root aphid or Pemphigus betae Doane
• Russian wheat aphid or Diuraphis noxia (Mordvilko)
Over the years, both the Weekly Updates and Insect of the Week have included aphid-related information but also important natural enemy details to support in-field scouting. Review the list below so pest and beneficial insects can be distinguished readily when scouting fields:
• Aphidius wasp (Insect of the Week; 2015 Wk15)
• Aphids in canola (Insect of the Week; 2016 Wk13)
• Aphids in cereals (Insect of the Week; 2017 Wk09)
• Cereal aphid manager APP (Weekly Update; 2021 Wk07) that presently is available only for iOS
• Ladybird larva vs. lacewing larva (Insect of the Week; 2019 Wk18)
• Ladybird beetles and mummies (Weekly Update; 2020 Wk15)
• Lygus bug nymphs vs. aphids (Insect of the Week; 2019 Wk16)
• Hoverflies vs. bees vs. yellow jacket wasps (Insect of the Week; 2019 Wk19)
• Pea aphids: a persistent problem for legume growers (Insect of the Week; 2021 Wk12)
• Soybean aphids and aphid annihilating allies (Insect of the Week; 2022 Wk07)
• Syrphid flies (Insect of the Week; 2015 Wk16)