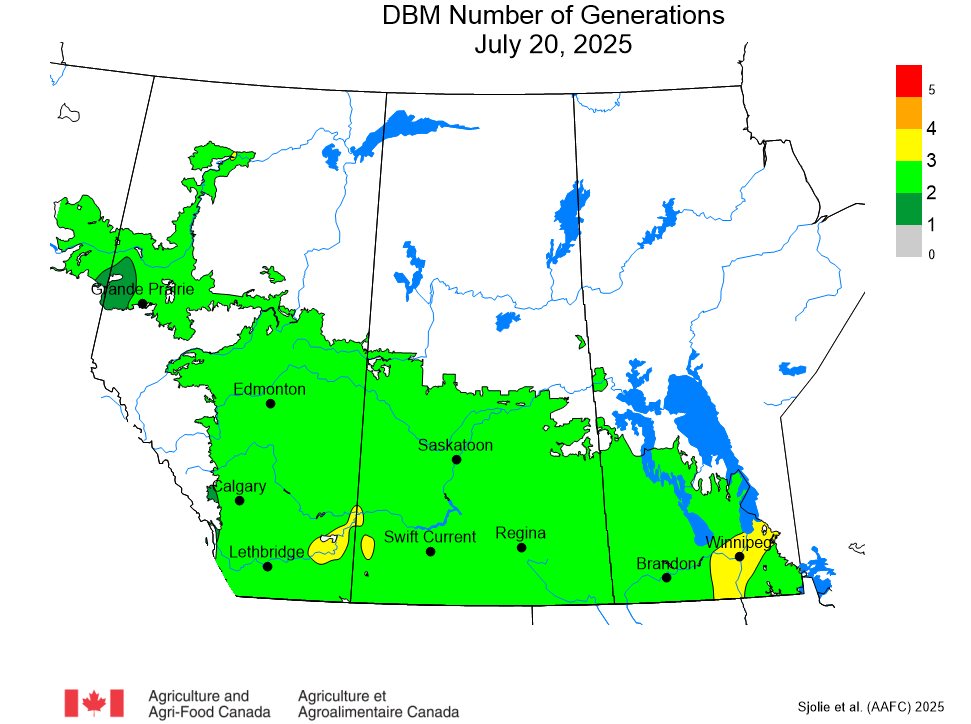
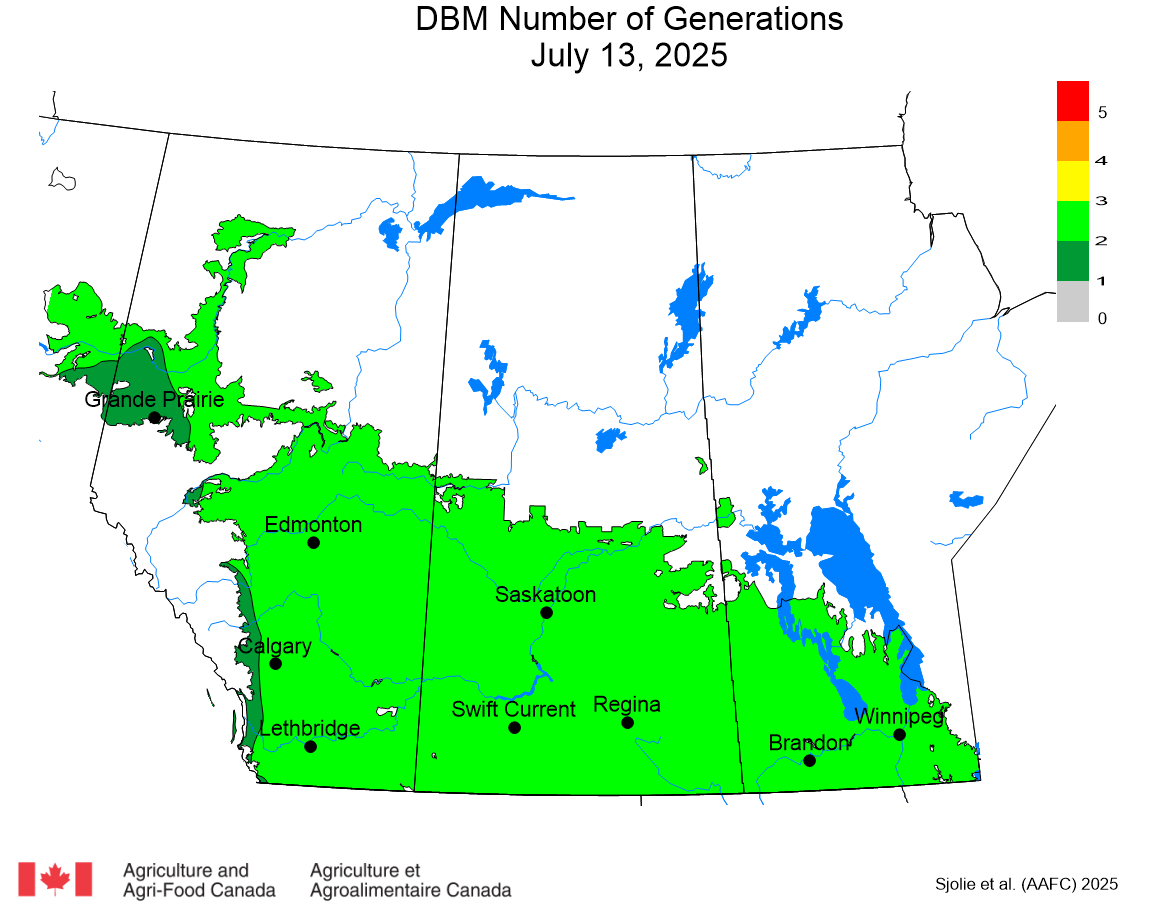
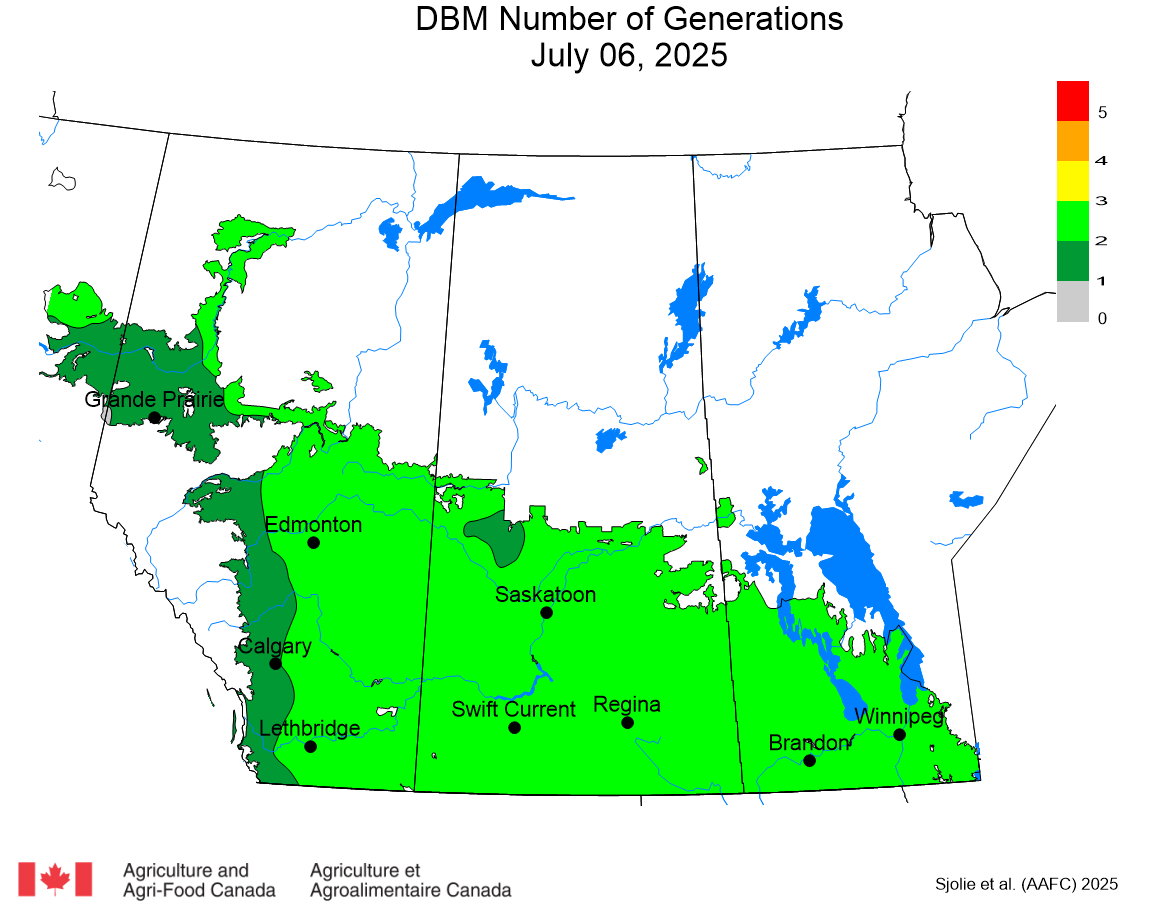
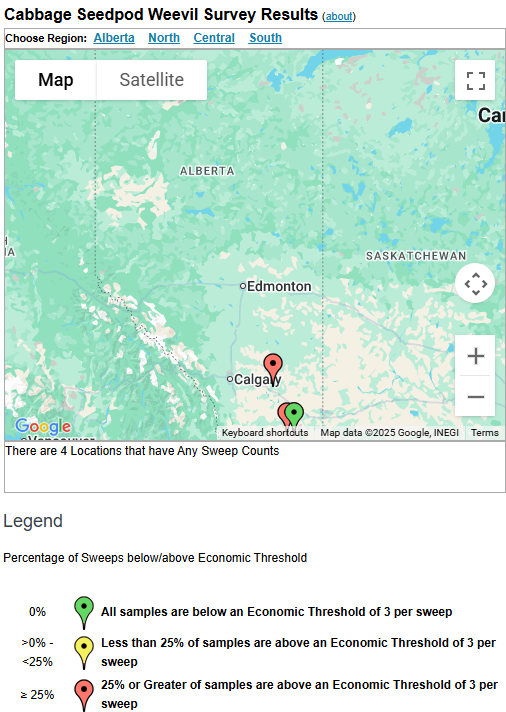
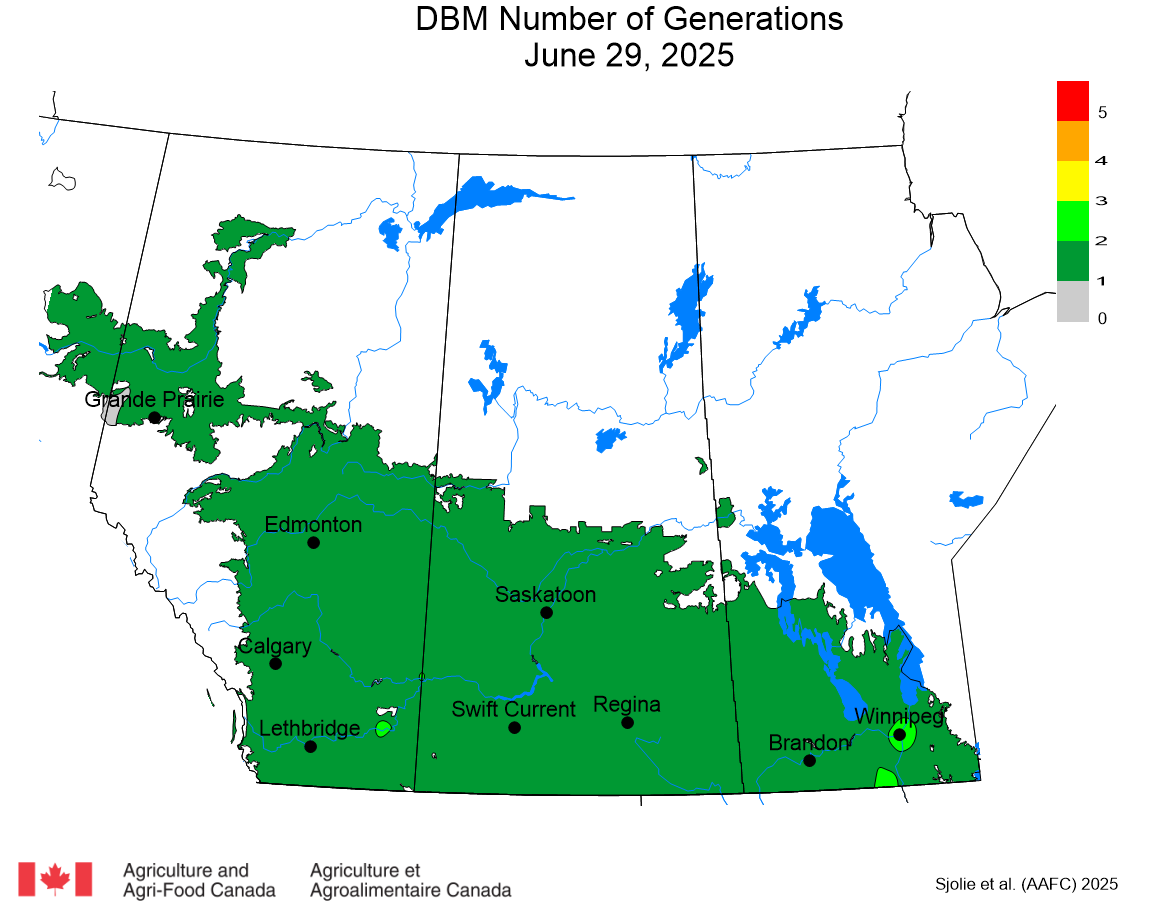
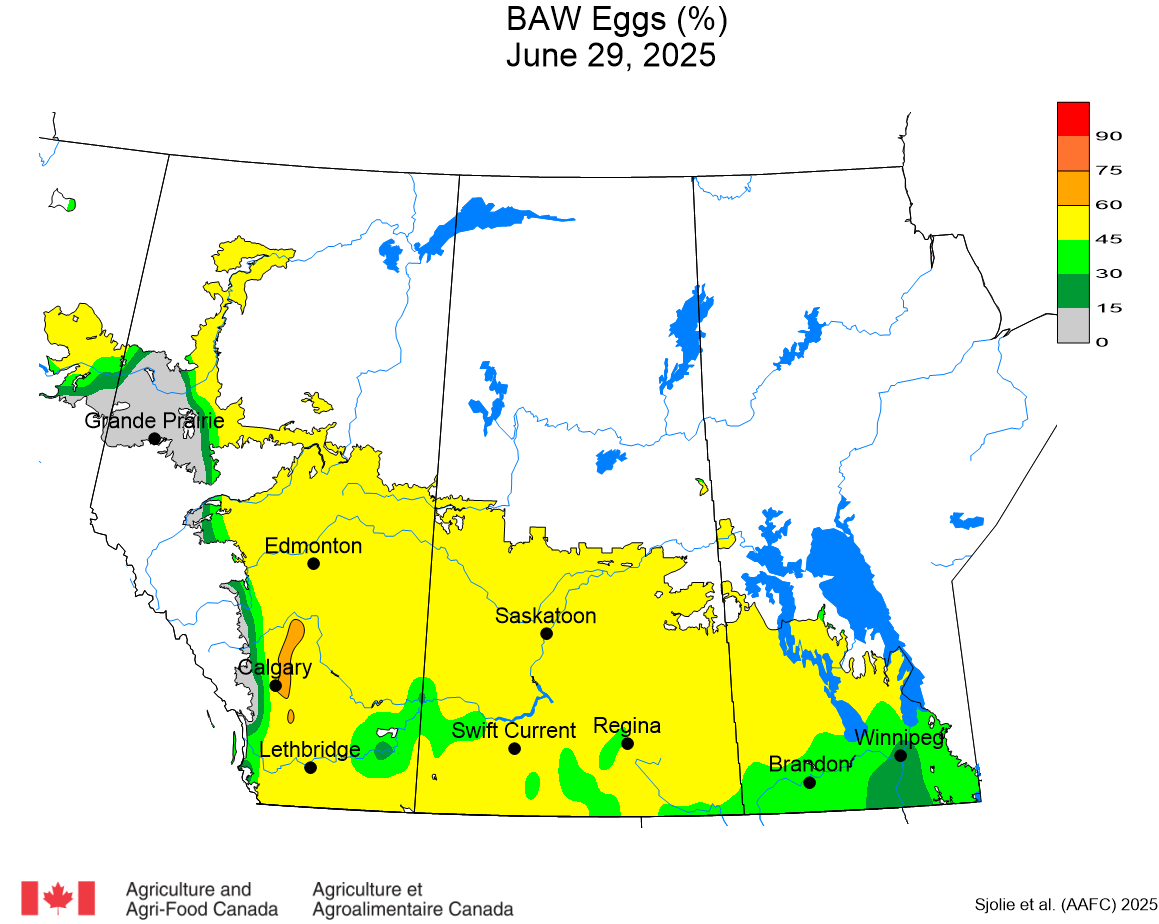
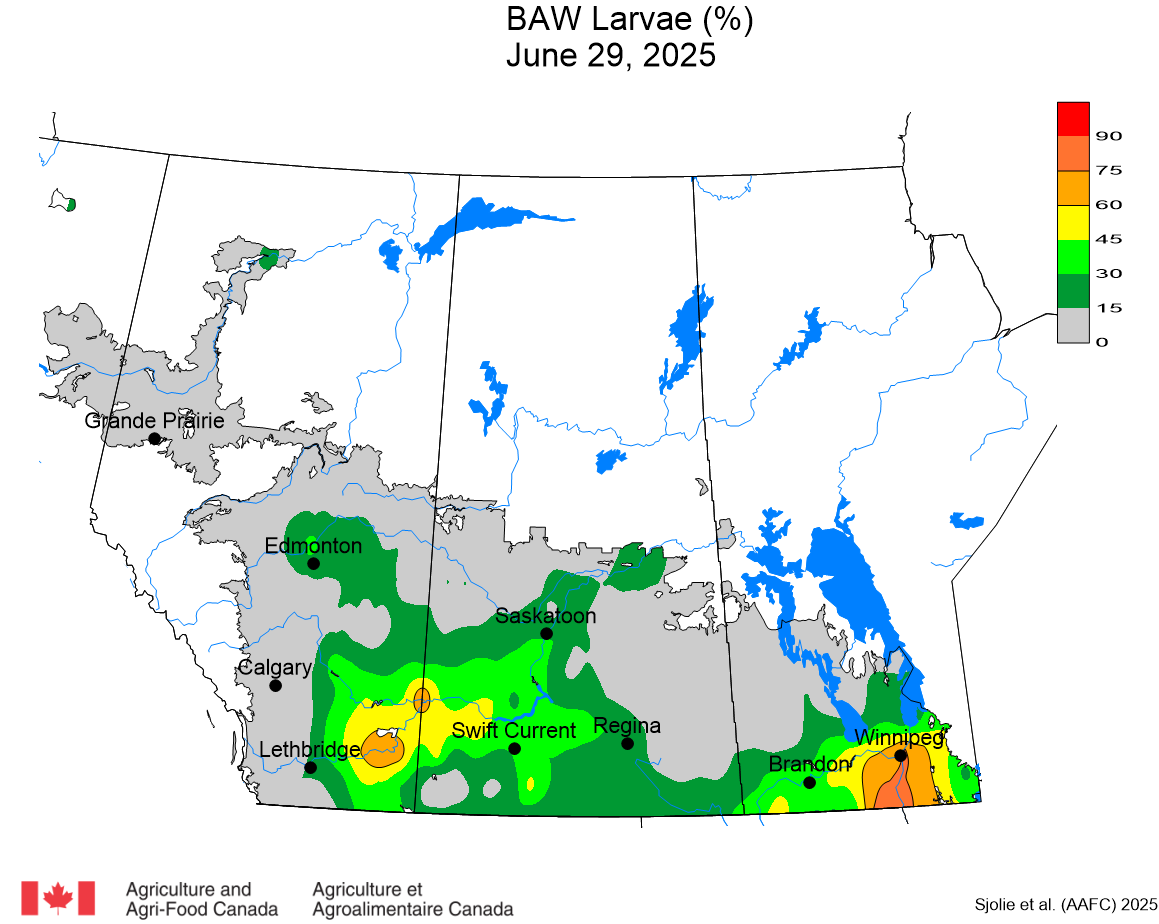
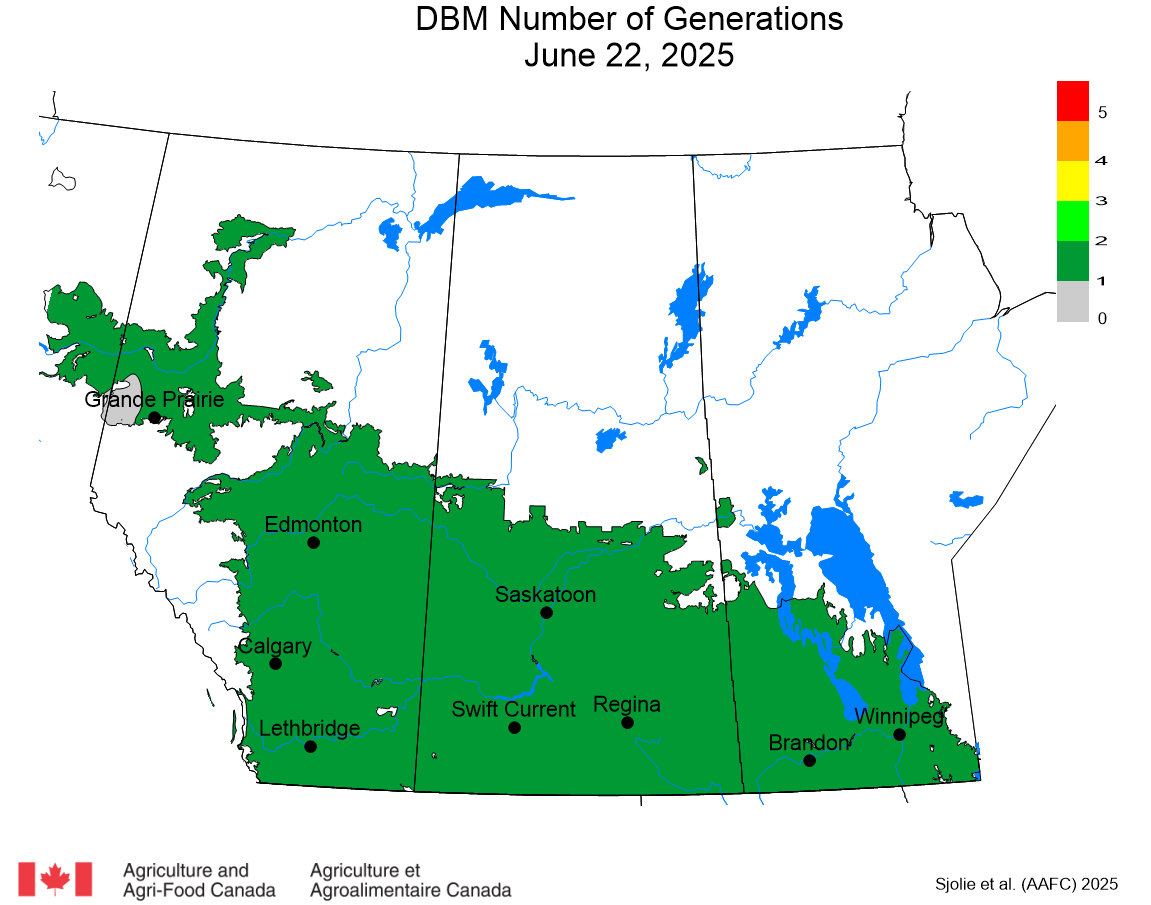
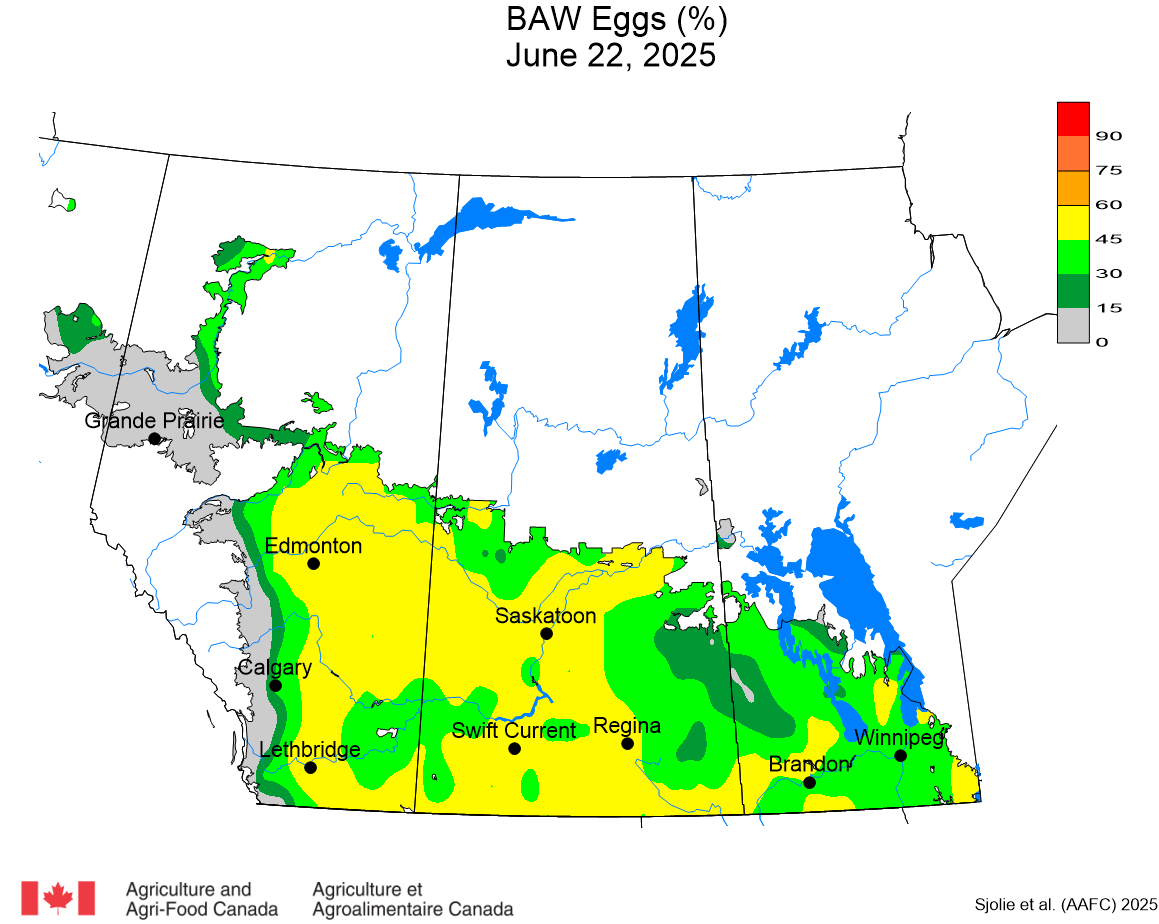
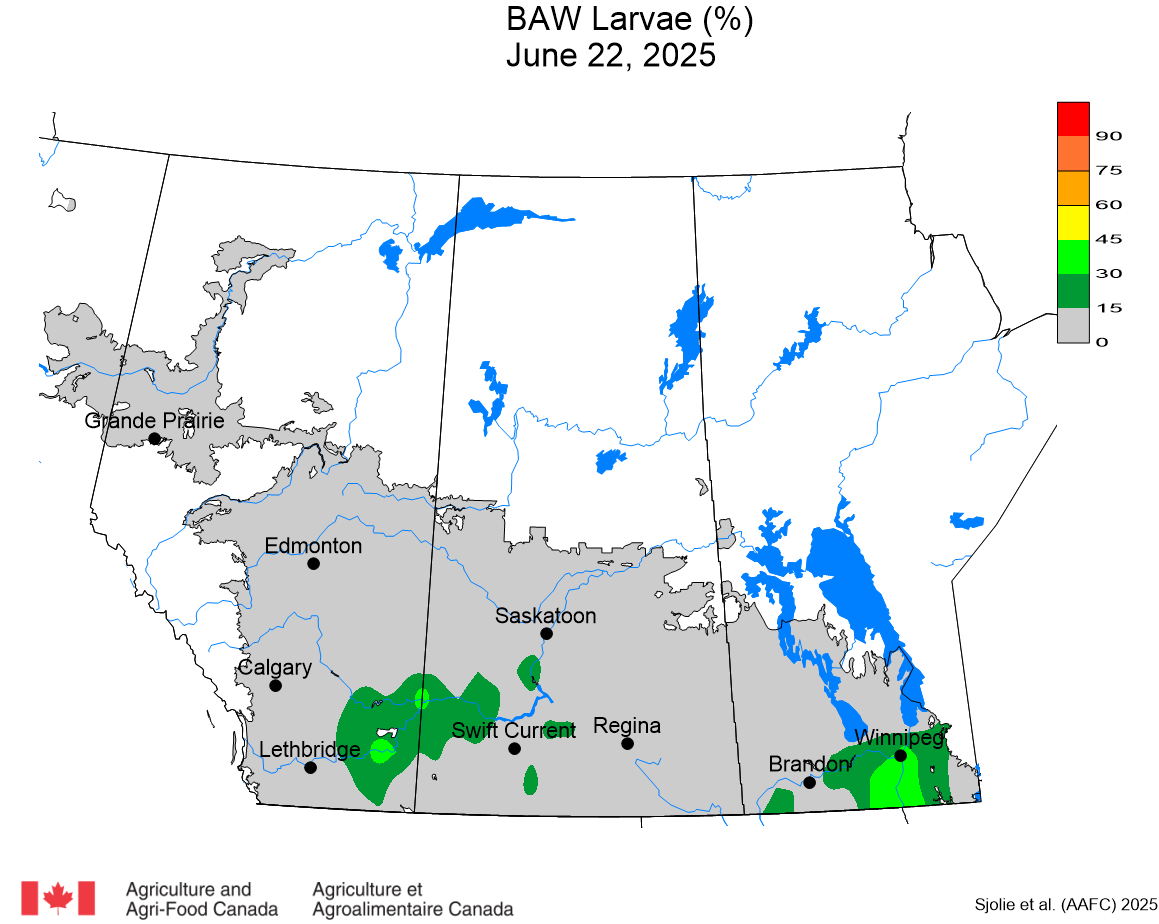
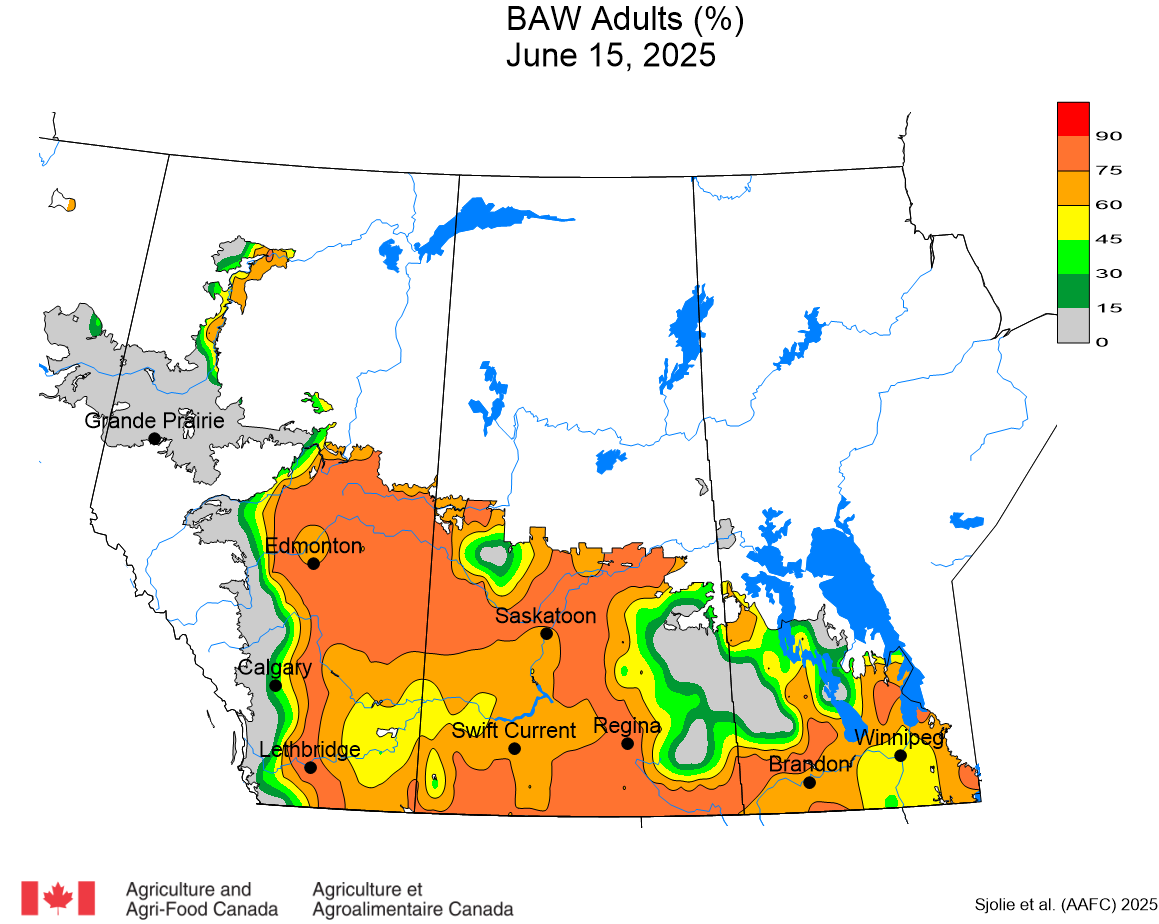
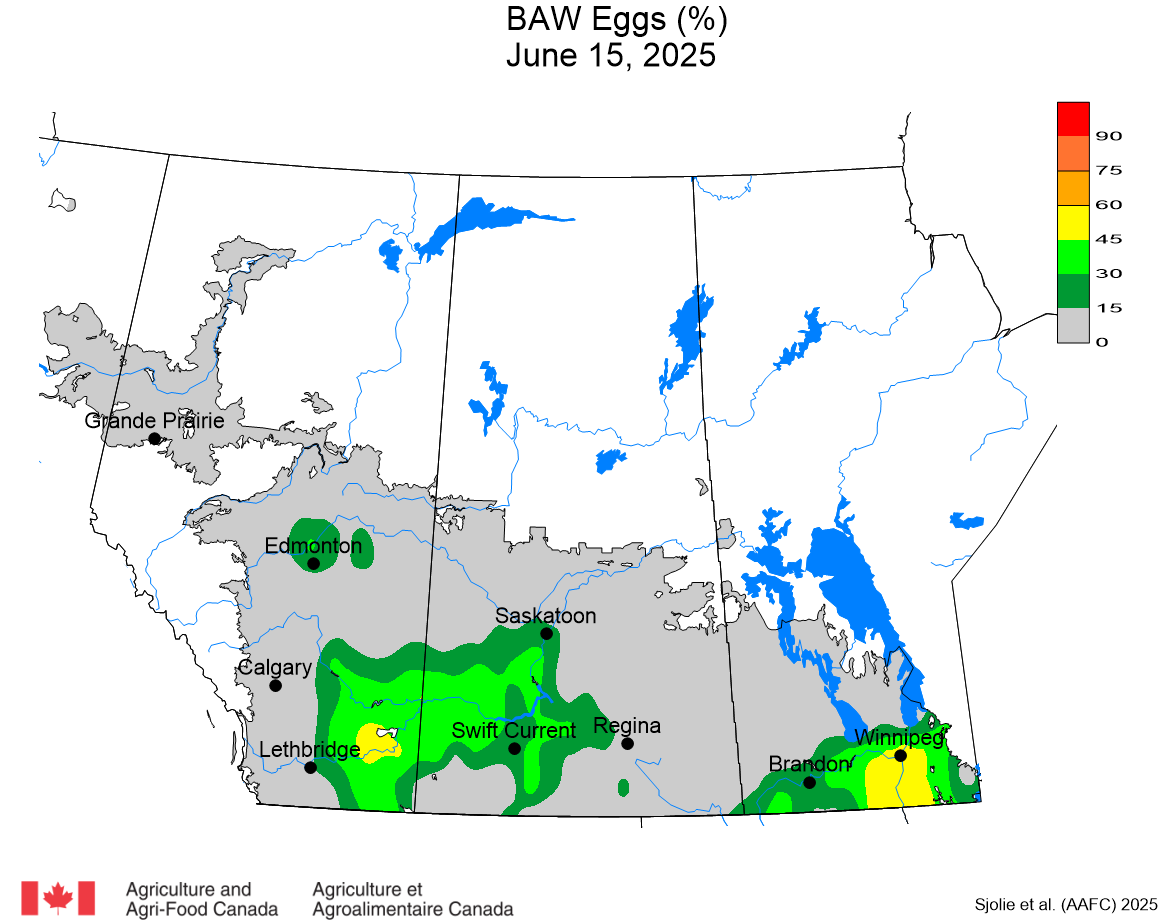
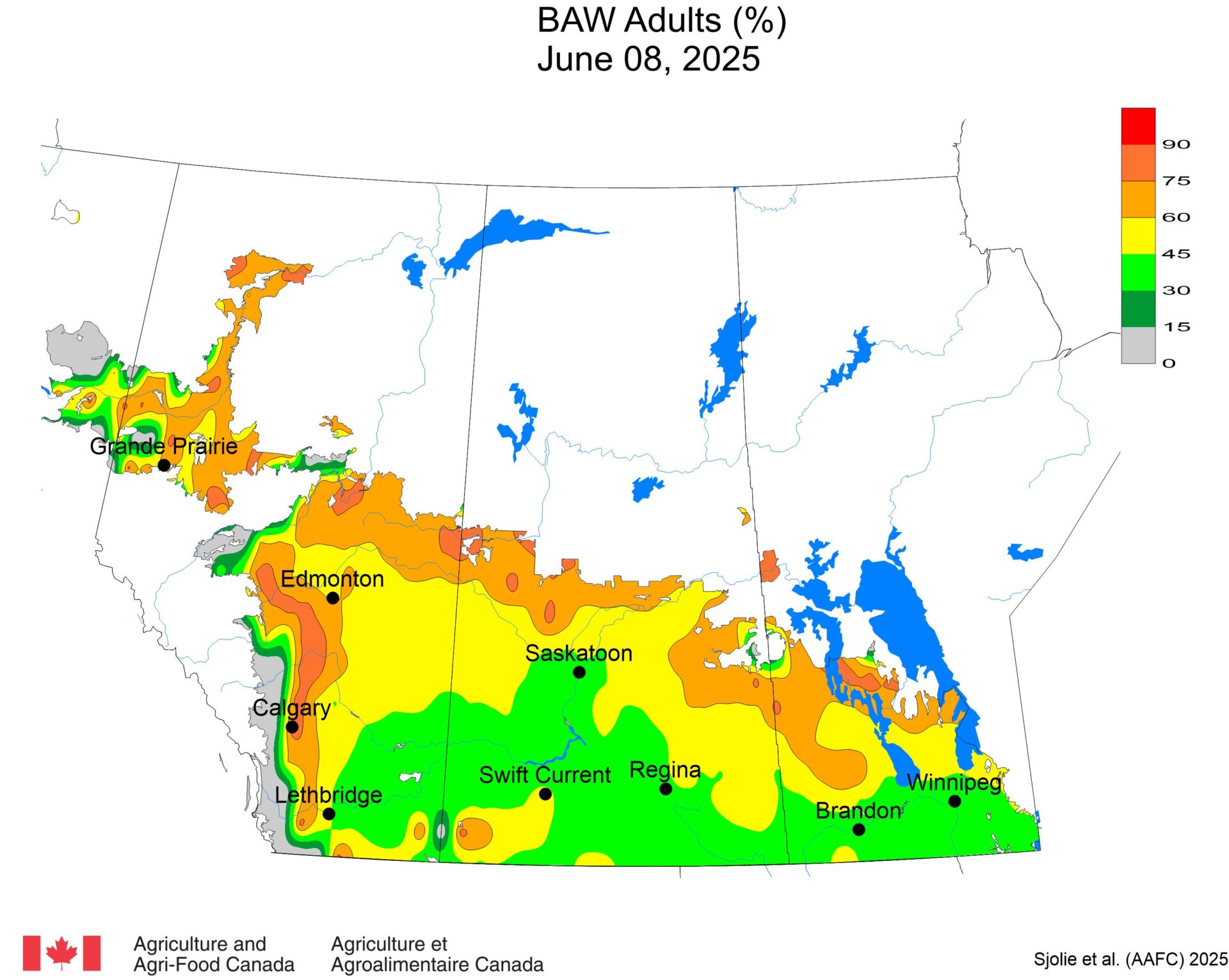
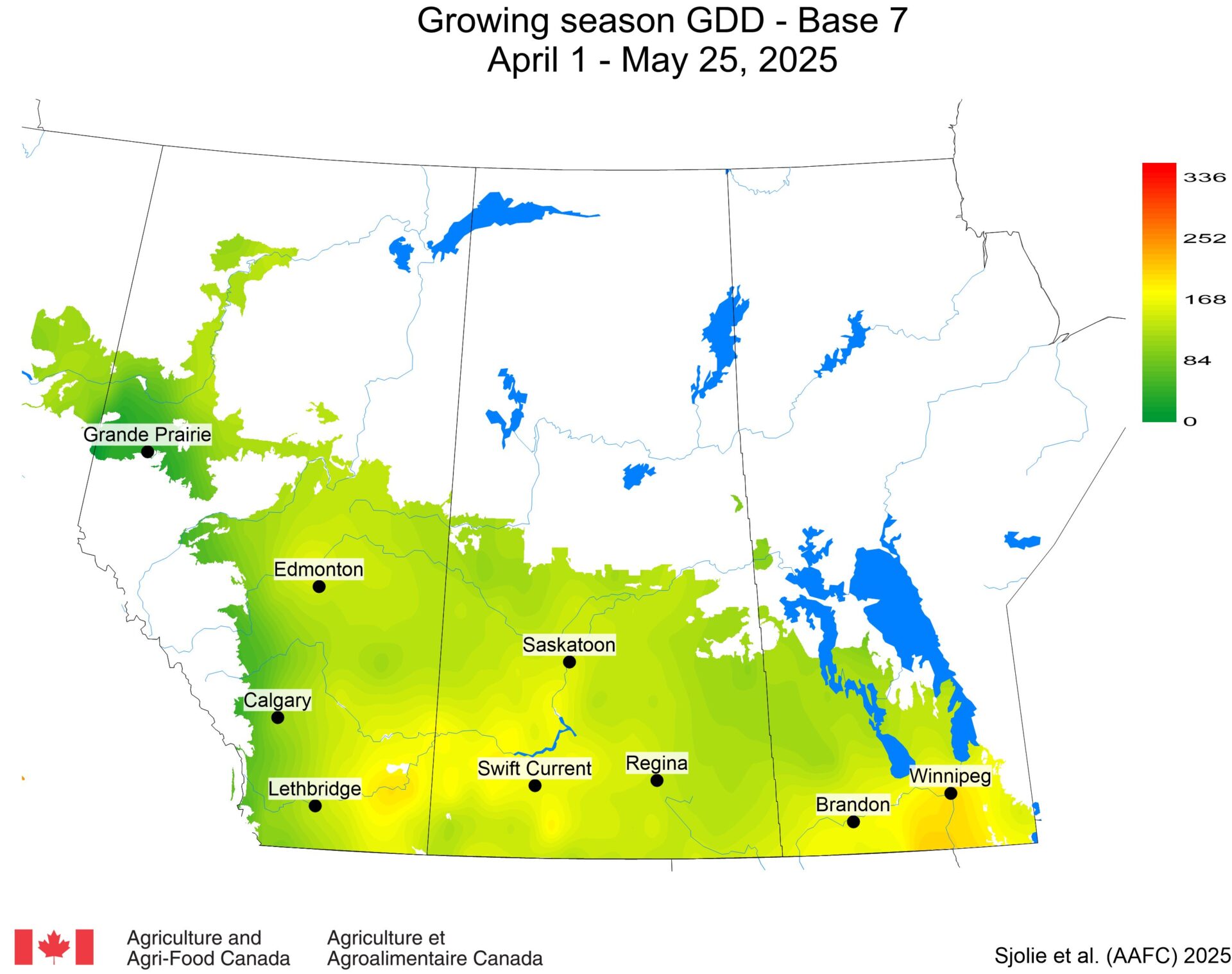
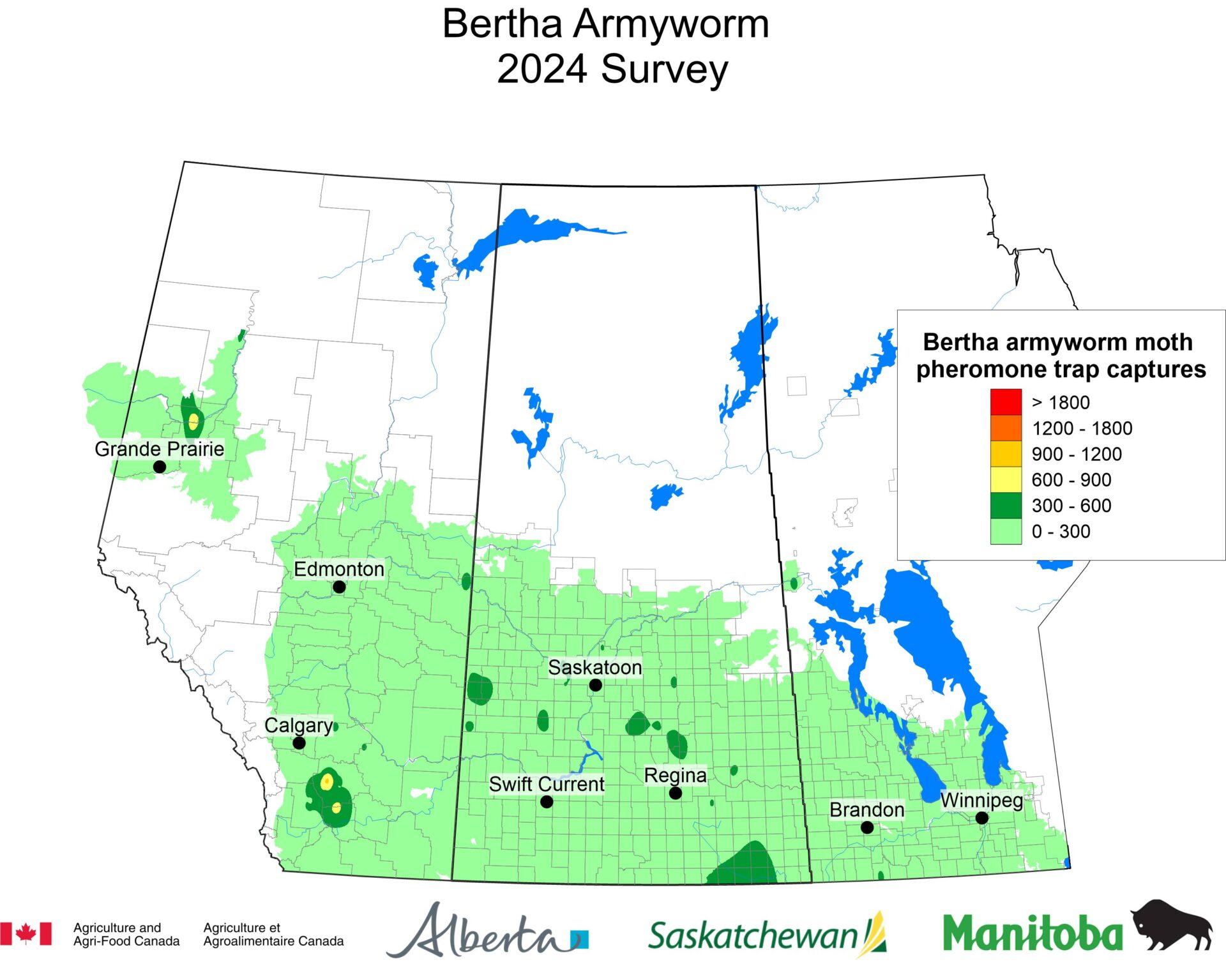
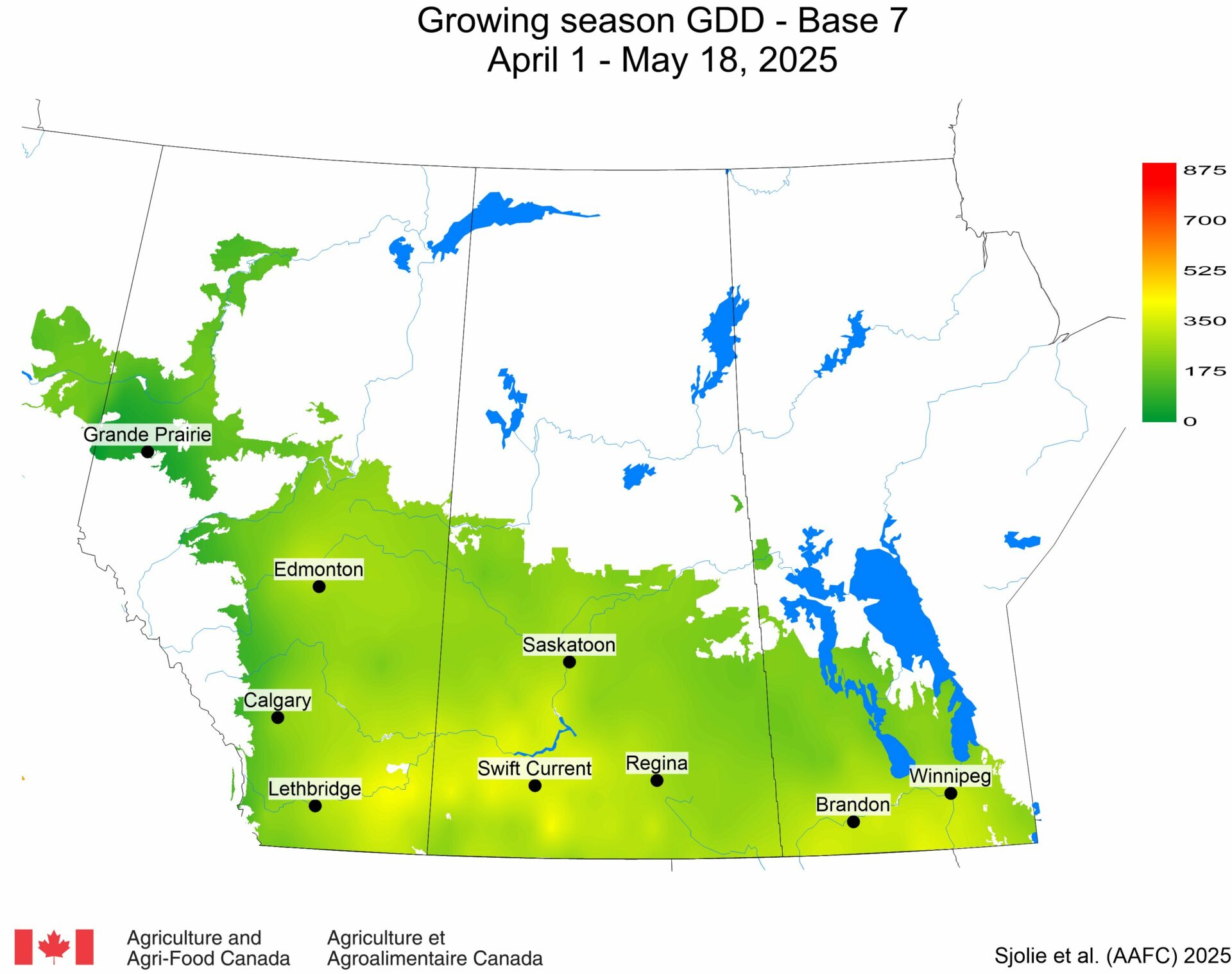
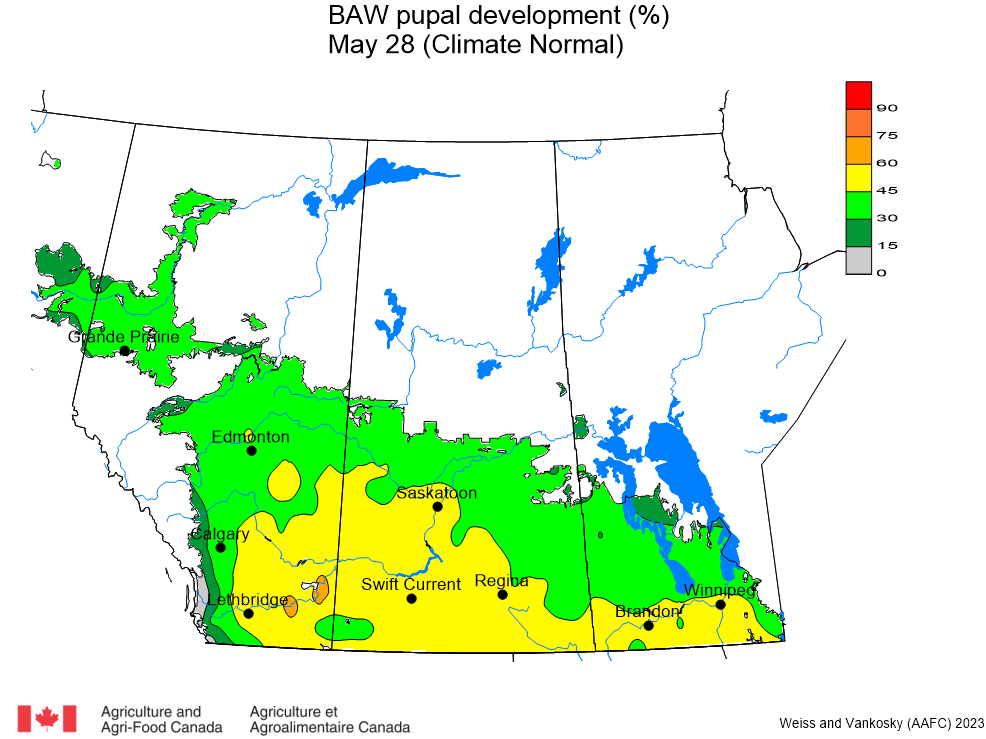
The phenology model for bertha armyworm development on the Canadian prairies was developed by Ross Weiss and Owen Olfert. Model simulations used to estimate development of bertha armyworm are now complete for the 2025 growing season. Now, in-field scouting for larvae is important!
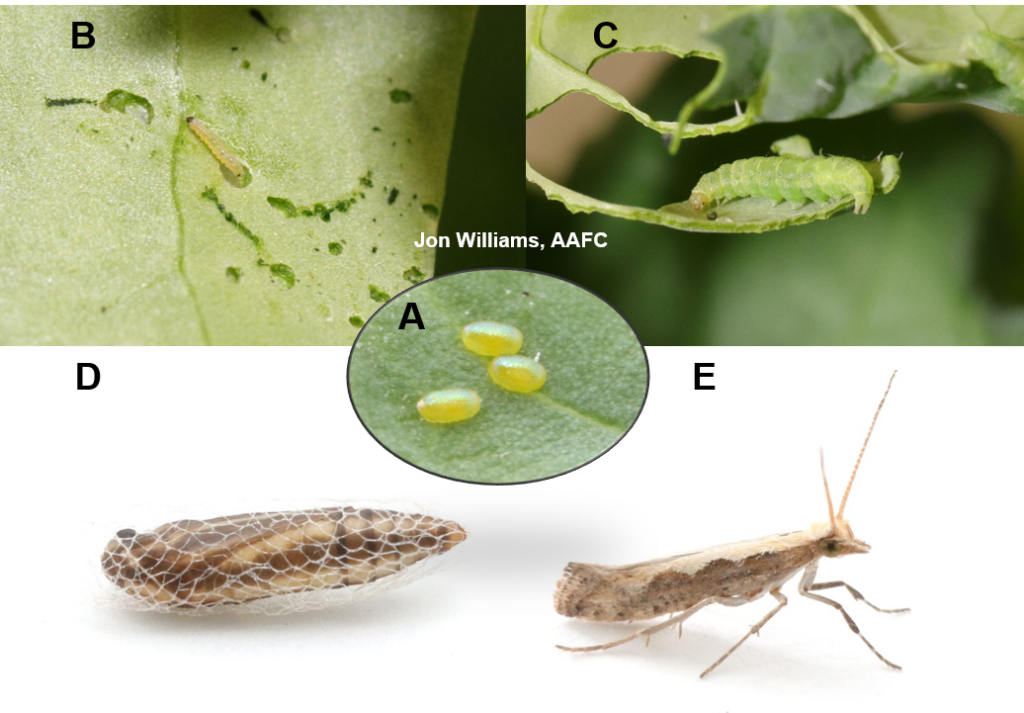
Figure 1 includes photos of the various life stages of the bertha armyworm. There is one generation per year and pupae overwinter in the soil (Fig. 1, C). Each growing season, green unitraps utilizing pheromone lures are deployed and checked weekly over a 6-week window. Cumulative counts generated from the pheromone traps are used to estimate subsequent bertha armyworm densities. The cumulative moth count data is compiled using geospatial maps then posted to support and time in-field scouting for damaging populations of larvae by mid-July through to August.

Please refer to this week’s Provincial Insect Pest Report Links to find the most up-to-date information summarizing weekly cumulative counts compiled by provincial pheromone trapping networks across the Canadian prairies in 2025. For example, Manitoba Agriculture’s June 19th Crop Pest Report includes Figure 2 with a reminder that other moth species are actively flying now so examine wing colourations and patterning carefully when checking the contents of bertha armyworm pheromone traps! Clover cutworm can be common by-catch in pheromone traps designed to monitor bertha armyworm, but also those designed to monitor true armyworm.
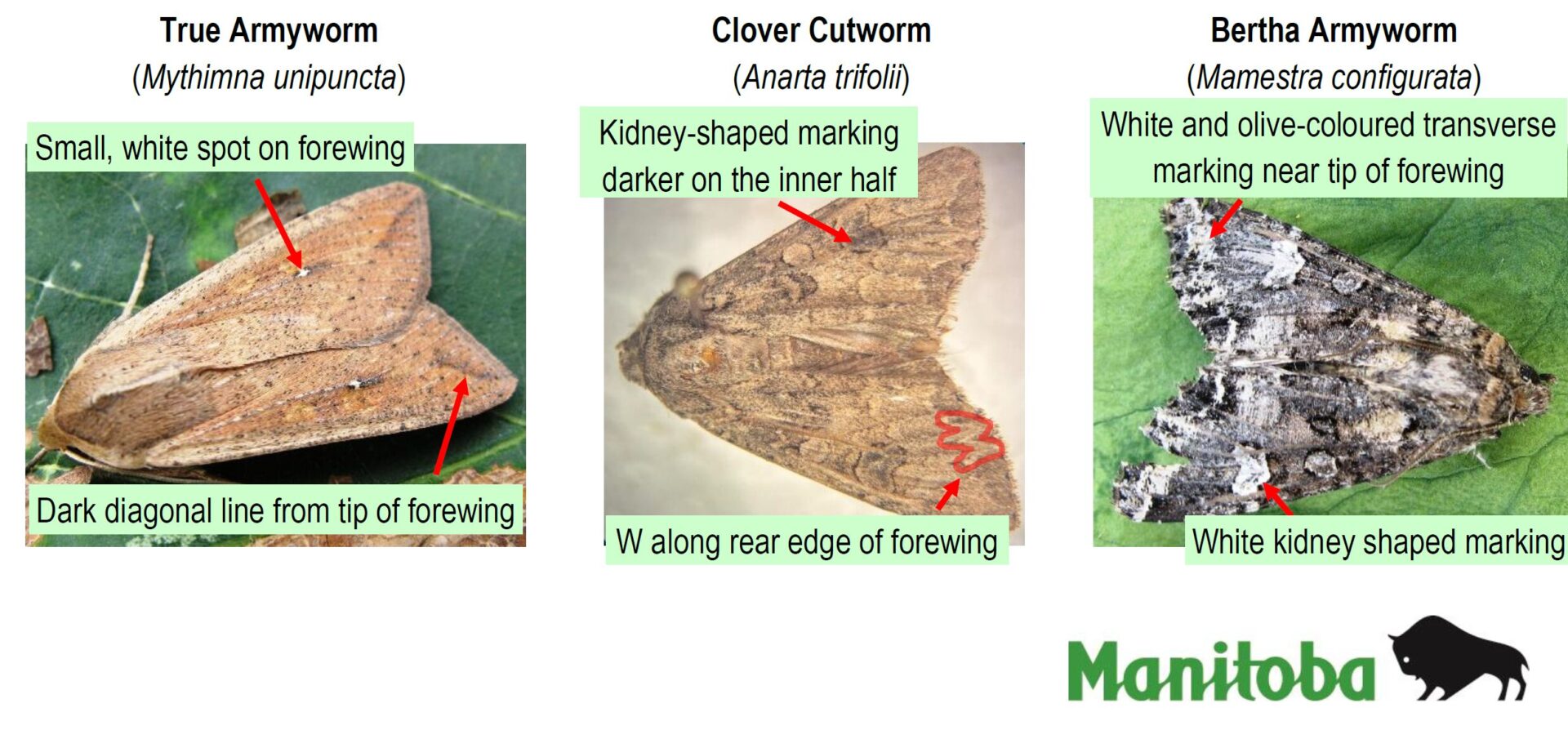
Biological and monitoring information related to bertha armyworm in field crops is posted by the provinces of Manitoba, Saskatchewan, Alberta and the Prairie Pest Monitoring Network. Also, refer to the bertha armyworm pages within the “Field Crop and Forage Pests and their Natural Enemies in Western Canada: Identification and management field guide” (2018), accessible as a free downloadable PDF in either English or French on our Field Guides page. Also consider reviewing this 2019 Insect of the Week featuring bertha armyworm and its doppelganger, the clover cutworm!